- Department of Neurosurgery, Centro Hospitalar Universitário do Algarve, Faro, Portugal
- Department of Anatomical Pathology, Centro Hospitalar Universitário de Lisboa Central, Lisboa, Portugal
- Department of Radiology, Centro Hospitalar Universitário do Algarve, Faro, Portugal.
Correspondence Address:
Pedro Gonçalo Abreu, Department of Neurosurgery, Centro Hospitalar Universitário do Algarve, Faro, Portugal.
DOI:10.25259/SNI_558_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pedro Gonçalo Abreu1, Lia Pappamikail1, Carlos Pontinha2, José Drago3, José Artur Lourenço1, Clara Romero1, Pedro Teles1, Joaquim Pedro Correia1. Case report: Rare convexity meningeal chondroma mimicking a meningioma. 24-Aug-2021;12:426
How to cite this URL: Pedro Gonçalo Abreu1, Lia Pappamikail1, Carlos Pontinha2, José Drago3, José Artur Lourenço1, Clara Romero1, Pedro Teles1, Joaquim Pedro Correia1. Case report: Rare convexity meningeal chondroma mimicking a meningioma. 24-Aug-2021;12:426. Available from: https://surgicalneurologyint.com/surgicalint-articles/11056/
Abstract
Background: Intracranial chondromas account for 0.2–0.3% of all intracranial neoplastic lesions and less than a quarter arise in the convexity or falx. Despite its benign nature, exceedingly rare malignant transformations exist. The misdiagnosis with meningiomas is frequent and may be related with chondromas’ similar insidious clinical presentation and imaging features. Standalone surgery is advised and complete resection provides the definitive treatment.
Case Description: A 44-year-old female presents with insidious headache, visual disturbances, and papilledema. The imaging studies were compatible with frontal parasagittal meningioma. Surgery revealed a meningeal based mass, mostly avascular and with a well-demarked surgical plane from the brain parenchyma. Complete resection with meningeal margins was achieved and the histopathologic examination revealed a chondroma. The patient symptoms subsided and no surgical complications existed.
Conclusion: Intracranial convexity chondromas constitute a rare differential diagnosis for meningiomas. The present case reinforces the current scarce data and serves as reminder for clinicians diagnosing and treating intracranial tumors.
Keywords: Case report, Meningioma, Chondroma, Chondrosarcoma
INTRODUCTION
Intracranial chondromas (ICCs) account for 0.2–0.3% of all intracranial neoplastic lesions.[
ICCs have similar gender distribution and most are diagnosed between the second and fifth decades of age.[
Considered benign tumors, malignant transformation of ICC may occur in 2.3% of the cases. Most of the cases described were associated with Maffucci syndrome.[
Surgical total resection of ICC is advisable. Incomplete resections frequently lead to reoccurrence and chemotherapy or radiation is not valid alternatives. Reoccurrence rarely follows complete resections.[
CASE DESCRIPTION
A 44-year-old female with unremarkable medical history presents at our outpatients consultation with headache and visual disturbances described as “flashlights.” Apart from an incipient papilledema, no other findings existed.
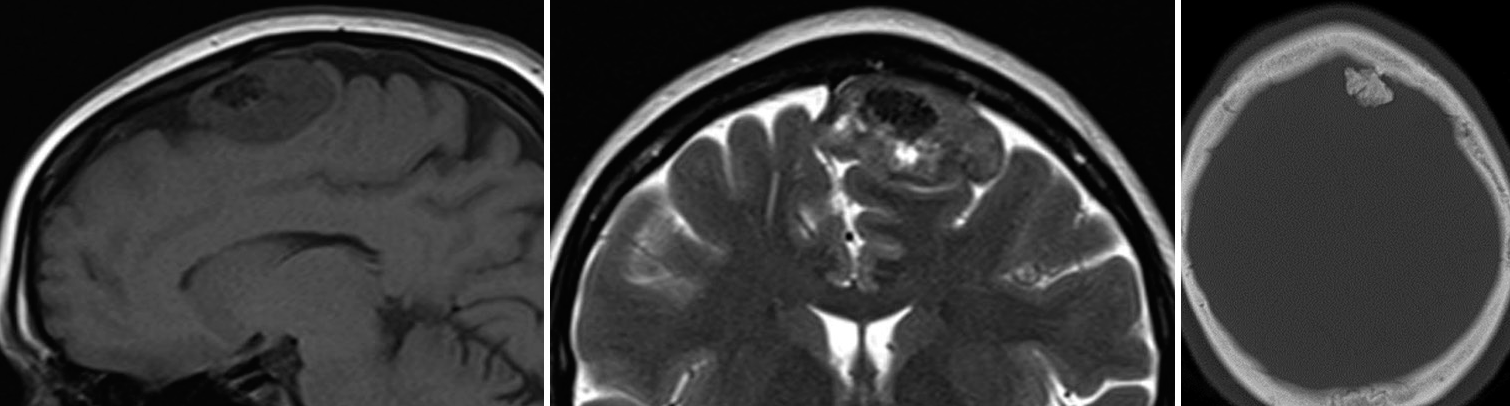
The CT scan revealed a left frontal parasagittal mass of 4 cm × 4 cm × 2 cm molding the surrounding brain parenchyma, mostly isodense, with a large central calcification and hyperostosis [
Figure 1:
Convexity chondroma magnetic resonance imaging (MRI) and computerized tomography (CT) images. On the left and middle captions, T1- and T2-weighted MRI images reveal a dural attached convexity mass, well demarked from brain parenchyma and with no associated edema. No apparent invasion of calvarium diploe is noticed. On the right, a CT scan bone window highlights the calcifications inside the mass, responsible for the hypointense behavior in T1 and T2 MRI images.
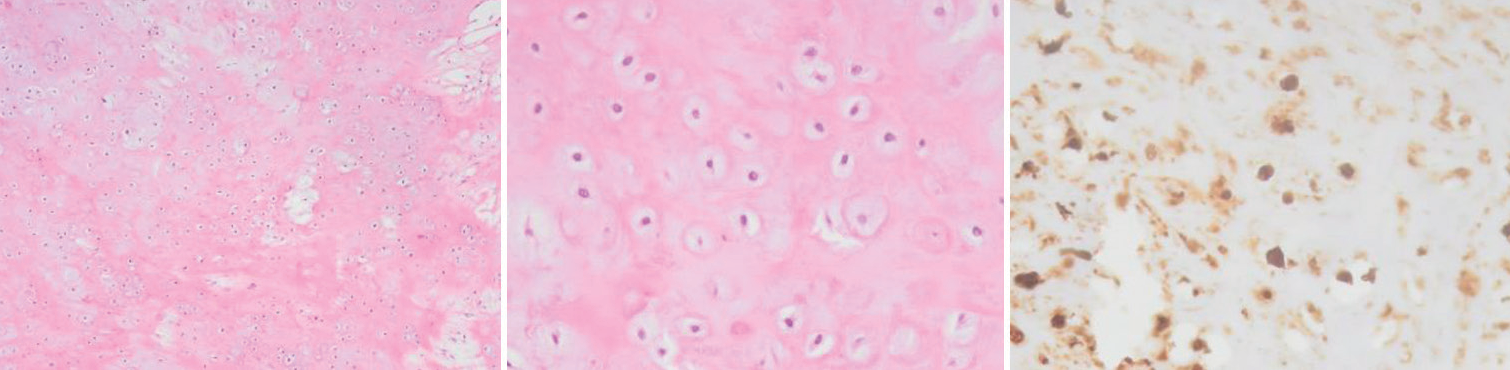
Surgical treatment was planned. Intraoperatively, a parasagittal mass pediculated from the convexity dura with lobular edges and a rubbery hard consistency was found. Its’ well-demarked margins and defined dissecting plan from the parenchyma allowed for a complete en bloc removal with dural margins. No postoperative complications or sequelae existed and the previous symptoms subsided. The histopathologic analysis revealed a mass of cartilaginous tissue without cytologic atypia, confirming the diagnosis of dural chondroma [
Figure 2:
Convexity chondroma on histology. Histopathological analysis reveals a monomorphous tumor of low cellularity. On the left and middle captions, we recognize chondrocytes without atypia embedded within a chondroid matrix. Immunohistochemically (on the right), neoplastic cells are S100 protein positive. In addition, they were vimentin positive and cytokeratin AE1/AE3, EMA, GFAP, and IDH1 negative (not shown). The Ki-67 labeling index was low (not shown).
DISCUSSION
The misdiagnosis of meningiomas in cases of chondromas is well described.[
When using CT studies, chondromas and most meningiomas appear as well-defined masses demarked from brain parenchyma. Furthermore, calcifications and hyperostosis are common in both cases.[
Almost all chondromas included in a recent systematic review followed surgical treatment.[
In the histopathological analysis, the differential diagnosis of chondrosarcoma must be excluded. Increased cellularity, mitosis, atypia (nuclei are hyperchromatic and enlarged), and infiltrating borders are characteristic of chondrosarcomas and not compatible with chondromas.[
CONCLUSION
Intracranial convexity chondromas are rare and can easily mimic meningiomas. The differential diagnosis is difficult or almost impossible before histopathologic confirmation. Our case underlines the need to keep this entity in mind since it will prone the decision to a surgical attitude due to its low but existent potential for malignancy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Futur Oncol. 2018. 14: 2161-77
2. Cosar M, Iplikcioglu AC, Bek S, Gokduman CA. Intracranial falcine and convexity chondromas: Two case reports. Br J Neurosurg. 2005. 19: 241-3
3. Duan F, Qiu S, Jiang J, Chang J, Liu Z, Lv X. Characteristic CT and MRI findings of intracranial chondroma. Acta Radiol. 2012. 53: 1146-54
4. Erdogan S, Zorludemir S, Erman T, Akgul E, Ergin M, Ildan F. Chondromas of the falx cerebri and dural convexity: Report of two cases and review of the literature. J Neurooncol. 2006. 80: 21-5
5. Erkan K, Beute G, Sluzewsky M, Rooij W, Teepen J. Giant chondroma of the falx. Case report and review of the literature. J Neurosurg. 1996. 85: 1161-4
6. Fountas KN, Stamatiou S, Barbanis S, Kourtopoulos H. Intracranial falx chondroma: Literature review and a case report. Clin Neurol Neurosurg. 2008. 110: 8-13
7. Hong JT, Lee SW, Son BC, Sung JH, Choi HC, Kim MC. Delayed occurrence of intracranial supratentorial chondroma following compound depressed skull fracture. Acta Neurochir (Wien). 2005. 147: 343-5
8. Nakasu S, Nakasu Y. Natural history of meningiomas: Review with meta-analyses. Neurol Med Chir (Tokyo). 2020. 60: 109-20
9. Nakayama M, Nagayama T, Hirano H, Oyoshi T, Kuratsu JI. Giant chondroma arising from the dura mater of the convexity; case report and review of the literature. J Neurosurg. 2001. 94: 331-4
10. Omezine S, Bouali S, Taallah M, Zehani A, Kallel J, Jemel H. Distinguishing falcine chondrosarcomas from their mimics and management. World Neurosurg. 2018. 118: 279-83
11. Somerset HL, Kleinschmidt-Demasters BK, Rubinstein D, Breeze RE. Osteochondroma of the convexity: Pathologicneuroimaging correlates of a lesion that mimics high-grade meningioma. J Neurooncol. 2010. 98: 421-6
12. Sullivan JC, Goldsmith J, Rojas R, Varma H, Kasper EM. Intracranial dural parafalcine chondroma: Case report and systematic review of the literature. World Neurosurg. 2019. 122: 1-7
13. Tanohata K, Maehara T, Aida N, Unimo S, Matsui K, Mochimatsu Y. Computed tomography of intracranial chondroma with emphasis on delayed contrast enhancement. J Comput Assist Tomogr. 1987. 11: 820-3
14. Živković N, Berisavac I, Marković M, Milenković S. Falx chondroma with hyperostosis of the scull: A case report. Srp Arh Celok Lek. 2014. 142: 464-7