- Department of Neurosurgery, Hospital Universitario Fundacion Jimenez Diaz, Madrid, Spain.
- Department of Neurosurgery, Hospital Quironsalud Zaragoza, Aragon, Spain.
- Department of Pathology, Hospital Universitario Fundacion Jimenez Diaz. Madrid, Spain.
DOI:10.25259/SNI_82_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andres Silva Montes de Oca1, Pablo Barbero-Aznarez2, Margarita Jo Velasco3, Monica Lara Almunia1. Cerebellar metastasis of a Liposarcoma: Case report and literature review. 21-Jun-2021;12:301
How to cite this URL: Andres Silva Montes de Oca1, Pablo Barbero-Aznarez2, Margarita Jo Velasco3, Monica Lara Almunia1. Cerebellar metastasis of a Liposarcoma: Case report and literature review. 21-Jun-2021;12:301. Available from: https://surgicalneurologyint.com/surgicalint-articles/10897/
Abstract
Background: Liposarcoma (LPS) is a rare type of tumor; they come from the adipose tissue. It is the most common type of soft-tissue sarcoma. Every type of LPS has morphological features, immunophenotypic, and molecular pathogenesis characteristics of their own. In this case, we are going to report a cerebellar metastatic disease from a well-differentiated liposarcoma (WDL) with pleomorphic component, not found in our literature research.
Case Description: A 72-year-old woman with progressive pain and inflammation in the left knee with functional limitation when climbing stairs. MRI shows a tumor in the vastus medialis of the left thigh. Pathology result was pleomorphic and WDL, Stage III and negative for MDM2 and CDK4. Extension study was carried out, finding nodular lesion in the right cerebellar hemisphere with mass effect and partial obliteration of the fourth ventricle, suspicious of distant disease.
Conclusion: Cerebellar metastasis of LPS is uncommon; there are only a few cases reports with the literature reviews describing orbital or skull base metastases, but not in the cerebellum. Our case allows us to remember that neurological symptoms, no matter how subtle, in patients diagnosed with LPS, a secondary affectation of the central nervous system must be ruled out, even though it is a rare location. The findings of distant disease in LPSs, allow planning oncological treatment options and targeted radiotherapeutic.
Keywords: Adipose tissue, Cerebellum, Liposarcoma, Metastatic dissemination, Radiotherapy
INTRODUCTION
Liposarcoma (LPS) is a rare of tumor; they come from the adipose tissue. It is the most common type of soft-tissue sarcoma. Comprises about 20% of malignant mesenchymal neoplasms.[
According to the World Health Organization is divided in: well-differentiated/dedifferentiated liposarcoma (WDL/DDL), myxoid/round-cell LPS, and pleomorphic liposarcoma (PLS).[
Every type of LPS has morphological features, immunophenotypic, and molecular pathogenesis characteristics of their own. In this case, we are going to report a cerebellar metastatic disease from a WDL LPS, a rare possibility, not found in our literature research.
CASE DESCRIPTION
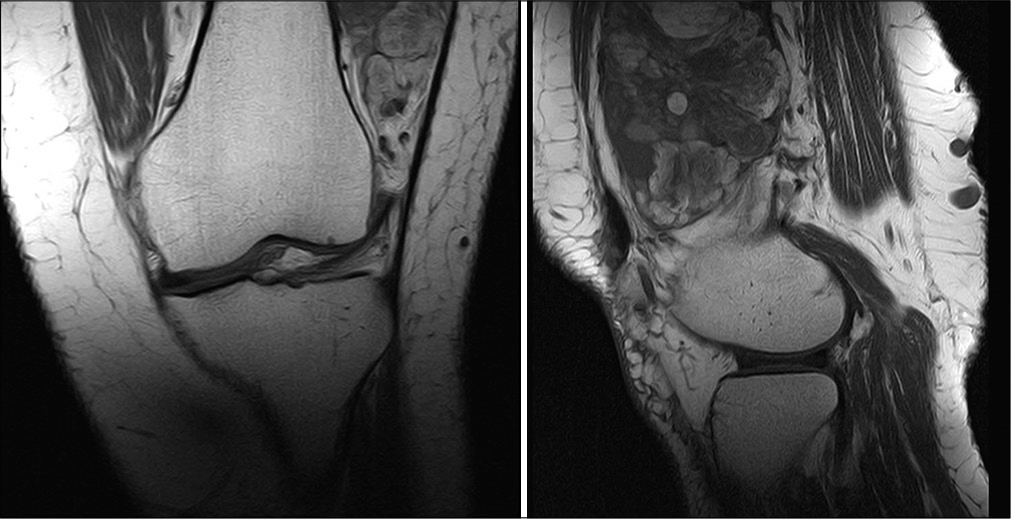
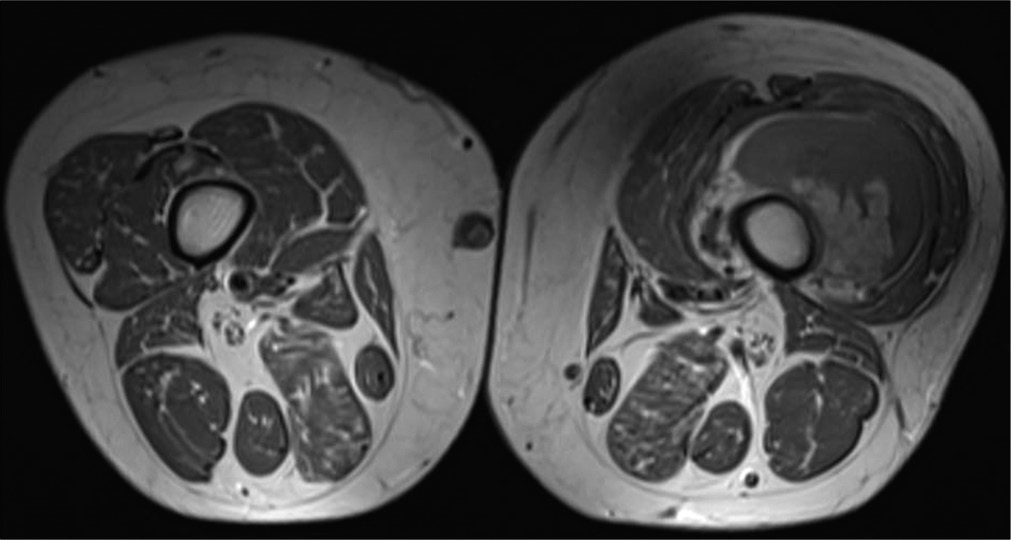
A 72-year-old woman, with the previous conditions of hypertension, diabetes, dyslipidemia, and chronic obstructive pulmonary disease, consulted for pain and progressive inflammation in the left knee with functional limitation when climbing stairs. Knee and thigh MRI [
Thick needle biopsy was made on the left femoral quadriceps, obtaining as result a WDL, MDM2, and CDK4 negative.
A wide surgical resection of left vastus lateralis muscle and vastus intermedius muscle was perform, with clear margins of resection. Pathology reports a high grade sarcoma (Grade 3) With a combined score 7 of the French system (FNCLCC) including: tumor differentiation 3, mitosis 3–50 mitosis in 10 CGA, necrosis 1). Cellularity with Dedifferentiation of pleomorphic and WDL. Histochemical study with MDM2 and CDK4 results negative for tumoral cells. Final staging: pT2b Nx Mx [
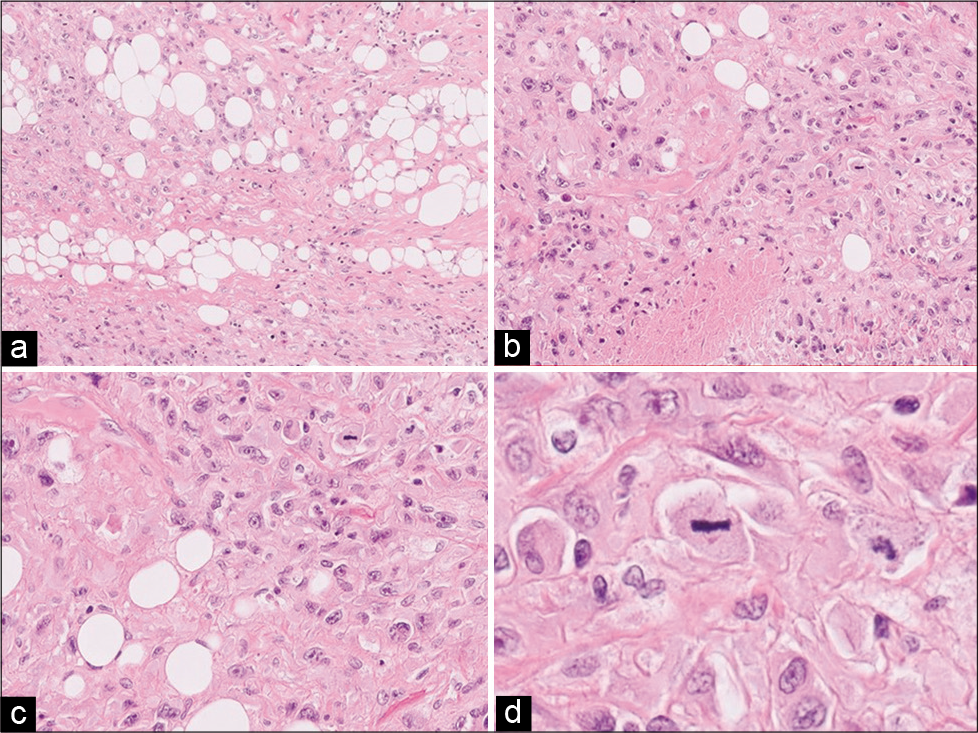
Figure 3:
Left thigh tumor pathology: (a) HE ×10: different areas were observed in the histological sections, some with a storiform pattern constituted with spindle, atypical, and polymorphic cells; also absence of differentiation and extensive necrosis. (b) HE ×20: atypical lipogenic cells were found in neoplastic cells, constituting a component of pleomorphic liposarcoma. (c) HE ×40 and (d) HE ×60: they continued with well-differentiated liposarcoma cells, with desmoplastic stroma, abundant lipoblastic cells, and hyperchromatic and pleomorphic nuclei and presence of numerous mitoses. Diagnosis: high-grade sarcoma (Grade 3. French system combined score 7 (FNCLCC): tumor differentiation 3, mitosis 3–50 mitoses in 10 CGA -, necrosis 1). (Dedifferentiation of pleomorphic and well-differentiated liposarcoma).
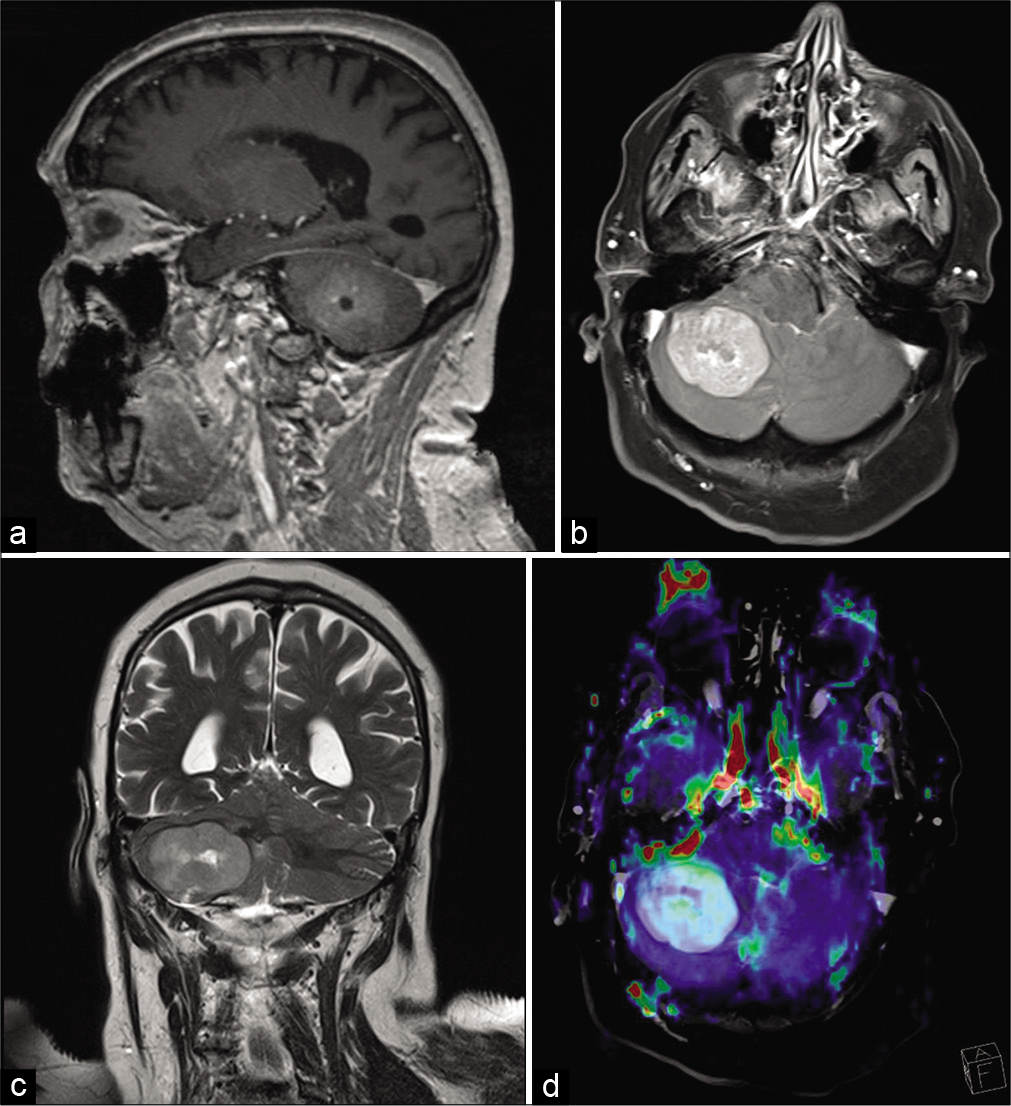
Two months after surgery, the patient complains of continuous headache, a head MRI was performed, finding a single nodular lesion in the right cerebellar hemisphere of approximately 4 x 3.5 cm, with mass effect in the posterior fossa and partial obliteration of the fourth ventricle. The lesion presents minimal edema in the medial region, peripheral restriction in diffusion sequences, and peripheral contrast uptake with cystic / necrotic central area. ADC minimum 0.8 mm2 /s. It presents a relative cerebral blood volume increase in perfusion sequence of 3.14. The dynamic perfusion curve shows a very significant recovery, above the baseline. Proposing a cerebellar metastasis as the first option [
A right retromastoid craniotomy was performed, with findings of a whitish tumor with a friable texture, suggestive of metastasis, proceeding to peripheral dissection of the tumor and alternated with internal tumor decompression until complete removal was achieved; the postoperative period was uneventful.
Regarding the cerebellar lesion, pathology reports a dedifferentiated tumor metastasis, sarcomatous aspect with fusiform pattern, marked nuclear atypia, high mitotic rate, and compatible with previous tumor metastasis [
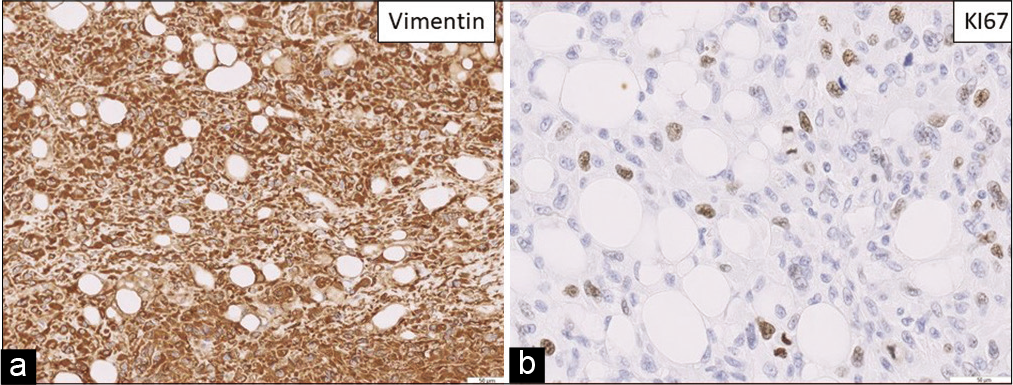
Figure 5:
(a) Vimentin 20X (b) KI67 40X. Cerebellar tumor: Dedifferentiated tumor metastasis, sarcomatous in appearance with a fusiform pattern, marked nuclear atypia, high mitotic rate, compatible with previous tumor metastasis. In the immunohistochemical study, it only expresses vimentin, with the absence of expression of the S100 protein and also the gliofibrillar protein and liposarcoma markers.
DISCUSSION
LPS are the most common type of soft-tissue sarcomas in adults. These tumors are typically detected in elderly people and appear especially in the lower extremities, abdomen, and shoulders.[
LPS can contain both well-differentiated mature adipocytes and initial undifferentiated cells; when they have more cells in earlier stages of differentiation, they behave more aggressive, and metastasize more frequently. However, the WDL can be dedifferentiated and be converted to DDL, achieving stronger invasive ability with potential local recurrence and distant metastasis. This dedifferentiation occurs in 10% of WDL.[
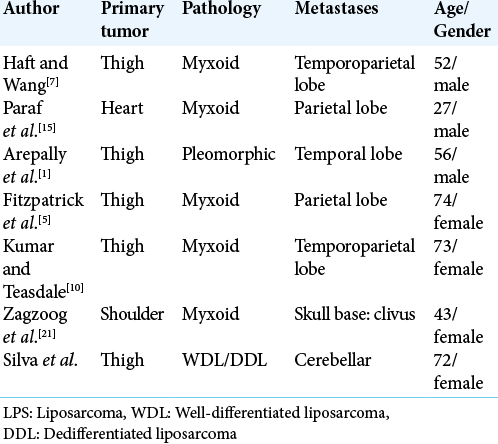
In case of LPS subtype WDL/DDL, there is no statistic describing the invasion of the CNS. There has been reported six patients with nonlymphomatous sarcoma metastatic to the brain,[
Prognosis is different for each type of cancer. Soft-tissue sarcomas, the prognosis is highly dependent on the tumor’s histological type.[
In addition to the histological result, the primary tumor must be radically resected with clean margins before adjuvant cancer treatment.[
RT has a role after brain metastases resection, either WBRT or SRS depending on the case; but in soft-tissue sarcomas, studies report adjuvant RT reduces the rate of local recurrences. However, the effectiveness of adjuvant RT in treating LPSs (DDL in particular) is still controversial; mostly because it does not modify the overall survival or the rate of metastasis.[
MRI of the total spine should be considered for myxoid/ round cell LPS due to the higher risk of metastasis to the spine compared to other soft-tissue sarcomas. CNS MRI (or CT if MRI is contraindicated) should be considered for patients with alveolar soft part sarcoma and angiosarcoma.[
According to NCCN guidelines, recommended follow-ups for soft-tissue sarcomas in patients with no radiographic evidence of disease must be: chest-abdominal and pelvic CT (or abdominal-pelvis MRI) every 3–6 months for 2–3 years; after the third year, the CT or MRI must be done every 6 month for the next 2 years; and after 5 years of follow-up, annually.
CONCLUSION
Cerebellar metastases secondary to a WDL LPS are infrequent; we did not find case reports or literature reviews. Our case recalls that appearance of neurological symptoms, no matter how subtle, in patients diagnosed with a soft-tissue sarcoma, a radiological image must be done. We consider that brain CT/MRI should be performed in patients with a previous diagnosis of LPS (regardless of whether the symptoms remit with medication) who present with headache, nausea/vomiting, neurological focality, seizure, and to rule out distant disease in the CNS. A quick diagnosis allows to plan early options for oncologic and multidisciplinary treatment.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arepally G, Kenyon LC, Lavi E. Late onset of isolated central nervous system metastasis of liposarcoma: A case report. Am J Clin Oncol. 1996. 19: 351-5
2. Briski LM, Jorns JM. Primary breast atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma. Arch Pathol Lab Med. 2018. 142: 268-74
3. Dei Tos AP. Liposarcoma: New entities and evolving concepts. Ann Diagn Pathol. 2000. 4: 252-66
4. Dei Tos A. Liposarcomas: Diagnostic pitfalls and new insights. Histopathology. 2013. 64: 38-52
5. Fitzpatrick MO, Tan K, Doyle D. Metastatic liposarcoma of the brain: Case report and review of the literature. Br J Neurosurg. 1999. 13: 411-2
6. Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F.editors. World Health Organization Classification of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2013. p.
7. Haft H, Wang GC. Metastatic liposarcoma of the brain with response to chemotherapy: Case report. Neurosurgery. 1988. 23: 777-80
8. Jo VY, Fletcher CD.editors. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology. 2014. 46: 95-104
9. Kim SE, Kim JH, Yang SW. Dedifferentiated transformation in metastatic liposarcoma of orbit and brain. Orbit. 2020. 39: 437-40
10. Kumar S, Teasdale E. Metastatic liposarcoma of the brain. Clin Radiol. 2000. 55: 406-8
11. Laurino L, Furlanetto A, Orvieto E, Dei Tos AP. Well-differentiated liposarcoma (atypical lipomatous tumors). Semin Diagn Pathol. 2001. 18: 258-62
12. Lewis AJ. Sarcomas metastatic to the brain. Cancer. 1988. 61: 593-601
13. Miller RW, Young JL, Novakovic B. Childhood cancer. Cancer. 1995. 75: 395
14. .editors. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Soft Tissue Sarcoma, Version 2. 2021. p.
15. Paraf F, Bruneval P, Balaton A, Deloche A, Mikol J, Maitre F. Primary liposarcoma of the heart. Am J Pathol. 1990. 3: 175-80
16. Patel SR, Burgess MA, Plager C, Papadopoulos NE, Linke KA, Benjamin RS. Myxoid liposarcoma: Experience with chemotherapy. Cancer. 1994. 74: 1265-9
17. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics 2021. CA Cancer J Clin. 2021. 71: 7
18. Singer S, Corson JM, Gonin R, Labow B, Ebelein TJ. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994. 219: 165-73
19. Utsunomiya A, Kinouchi H, Kayama T, Yoshimoto T. Distant metastasis of liposarcoma to the Dura and skull: A case report. Br J Neurosurg. 1999. 13: 520-2
20. Yang L, Chen S, Luo P, Yan W, Wang C. Liposarcoma: Advances in cellular and molecular genetics alterations and corresponding clinical treatment. J Cancer. 2020. 11: 100-7
21. Zagzoog N, Ra G, Koziarz A, Provias J, Sommer D, Almenawer SA. Metastatic liposarcoma of the skull base: A case report and review of literature. Neurosurgery. 2017. 80: 219-23