- Department of Neurosurgery, The University of Alabama at Birmingham, Birmingham, Alabama, United States.
- Department of Pathology, The University of Alabama at Birmingham, Birmingham, Alabama, United States.
Correspondence Address:
Nidal Bassam Omar
Department of Neurosurgery, The University of Alabama at Birmingham, Birmingham, Alabama, United States.
DOI:10.25259/SNI_616_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nidal Bassam Omar1, Gustavo Chagoya1, Galal A. Elsayed1, Silvio H. Litovsky2, James R. Hackney2, Winfield S. Fisher1. Cerebellar talcosis following posterior reversible encephalopathy syndrome in an intravenous methamphetamine abuser. 05-Jan-2021;12:2
How to cite this URL: Nidal Bassam Omar1, Gustavo Chagoya1, Galal A. Elsayed1, Silvio H. Litovsky2, James R. Hackney2, Winfield S. Fisher1. Cerebellar talcosis following posterior reversible encephalopathy syndrome in an intravenous methamphetamine abuser. 05-Jan-2021;12:2. Available from: https://surgicalneurologyint.com/surgicalint-articles/10514/
Abstract
Background: Intravenous (IV) methamphetamine abuse is associated with a variety of short- and long-term effects on the nervous system, some of which have yet to be fully elucidated. One known systemic complication that has not been described in nervous system tissues is the deposition of substrate crystals contained in injectable drugs.
Case Description: An unusual case is presented of a 35-year-old active IV methamphetamine abuser with posterior reversible encephalopathy syndrome (PRES) who subsequently developed multifocal bilateral cerebellar enhancing lesions and leptomeningeal enhancement due to biopsy-proven crystalline deposits.
Conclusion: Although large crystalline substances will not normally penetrate the blood–brain barrier (BBB), during a state of BBB compromise such as with PRES, talc deposition may occur in the central nervous system.
Keywords: Intravenous drug abuse, Methamphetamine, Positively birefringent crystals, Posterior reversible encephalopathy syndrome, Talc, Talcosis
INTRODUCTION
Intravenous (IV) drug abuse is a broad entity well known to perpetrate both acute and chronic insults that are deleterious to the complex physiology of the central nervous system (CNS). Psychostimulant drugs such as methamphetamines are recreationally abused through oral, inhalational, and IV routes. The latter often involves contamination with cutting or bulking substances including cornstarch and talcum powder (talc).[
CASE DESCRIPTION
A 35-year-old right-handed male with Type II diabetes mellitus, alcoholism, polysubstance abuse, posttraumatic stress disorder, and bipolar disorder presented to the emergency department (ED) with a 3-week history of progressive headaches, visual decline, and bilateral hand numbness. He admitted to using IV methamphetamines 3 weeks prior and to smoking cannabis nightly as a sleep aid. His vital signs and examination were noted to be normal except for the ED physician’s report that he “would not participate in visual fields examination.” His urine drug screen was positive only for cannabinoids. He underwent noncontrasted computed tomography (CT) as well as magnetic resonance imaging (MRI) of the brain demonstrating no abnormality, and he was subsequently discharged.
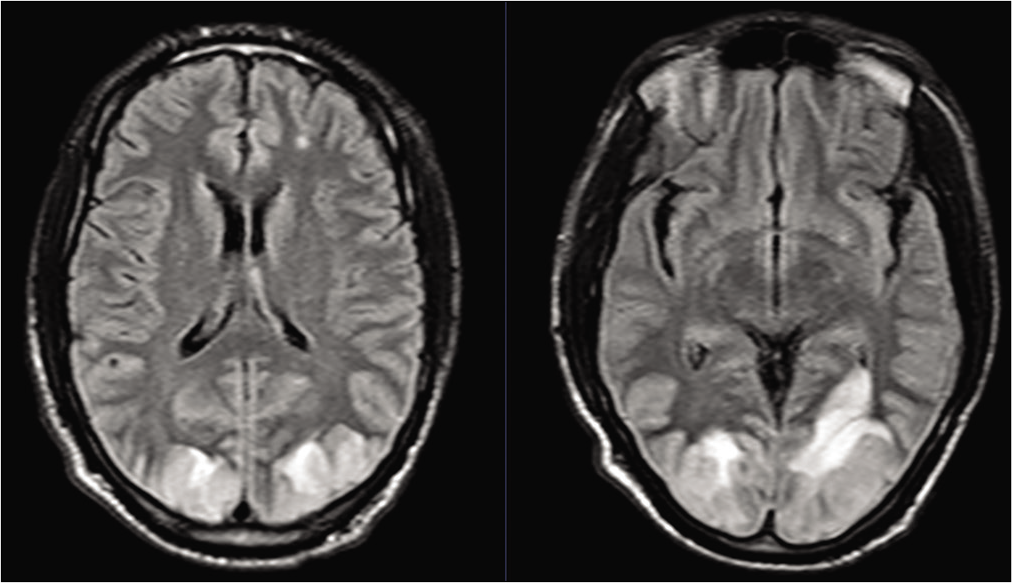
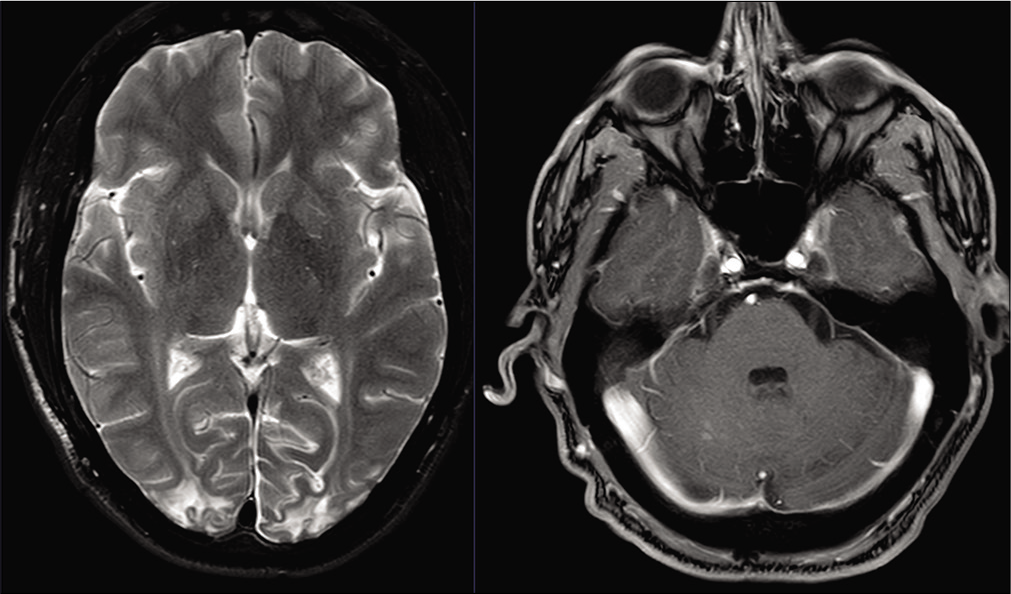
The patient represented to the ED 2 ½ weeks later with complaints of worsening headaches and visual loss along with nausea and photophobia. His vital signs were again noted to be normal and he was again noted to be uncooperative with a visual examination. CT on this occasion demonstrated bilateral occipital lobe hypodensities, and subsequent MRI imaging demonstrated bilateral occipital fluid-attenuated inversion recovery (FLAIR) changes with patchy diffusion restriction [
A consensus of the likely diagnosis of PRES was entertained. He was managed with headache control and repletion of mild hypomagnesemia. He was never found to have hypertension or require antihypertensive treatment. He continued to have difficult to control headaches, although he did report marginal subjective improvement in visual acuity. Given uncertainty regarding the etiology of his condition, digital subtraction angiography was performed and demonstrated no evidence of vasculitis or other cerebrovascular pathology. Ophthalmologic evaluation demonstrated no evidence of papilledema and visual acuity of 20/800 bilaterally. He underwent a lumbar puncture (LP) with an opening pressure of 28 cm H2O and cerebrospinal fluid (CSF) studies demonstrating a relative neutrophilia [
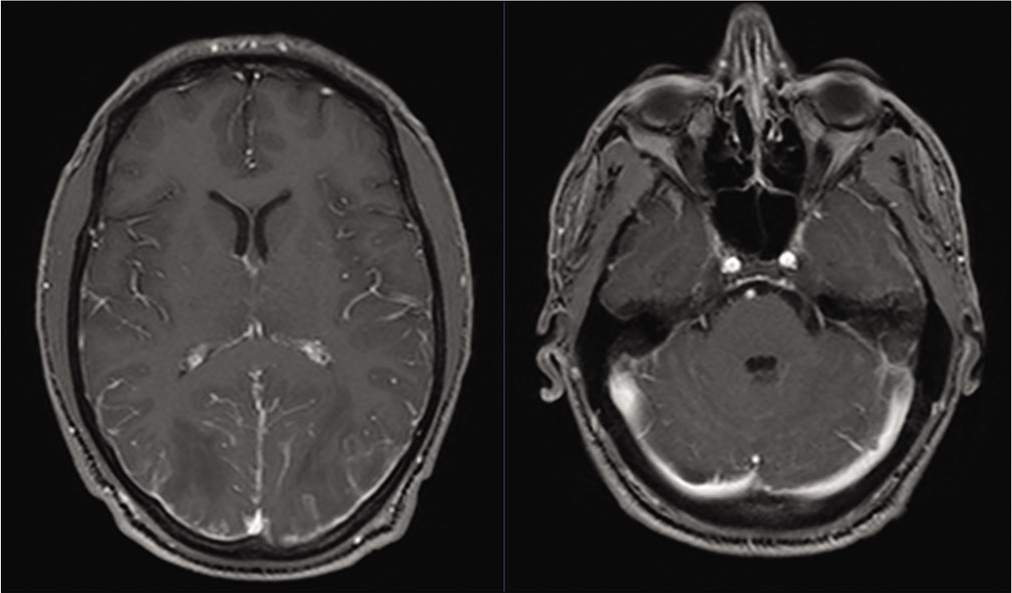
On the basis of these results and concern for meningitis, he was empirically started on broad-spectrum antimicrobial therapy which was later discontinued. CSF was sent for additional bacterial, viral, fungal, inflammatory, and autoimmune assays. Repeat MRI demonstrated only mild increase in the bilateral occipital lobe FLAIR changes but new extensive leptomeningeal enhancement [
He was started on IV Solumedrol due to concern for the possibility of an inflammatory process, which led to mild improvement in the patient’s headaches but not vision. Subsequent MRI was unchanged. Repeat LP revealed an opening pressure of 50 cm H20 and CSF with a lymphocytic predominance [
Two weeks after discharge, his headaches had improved as did his visual acuity to 20/70 on the left and 20/100 on the right. A noncontrasted head CT showed stable occipital hypodensities.
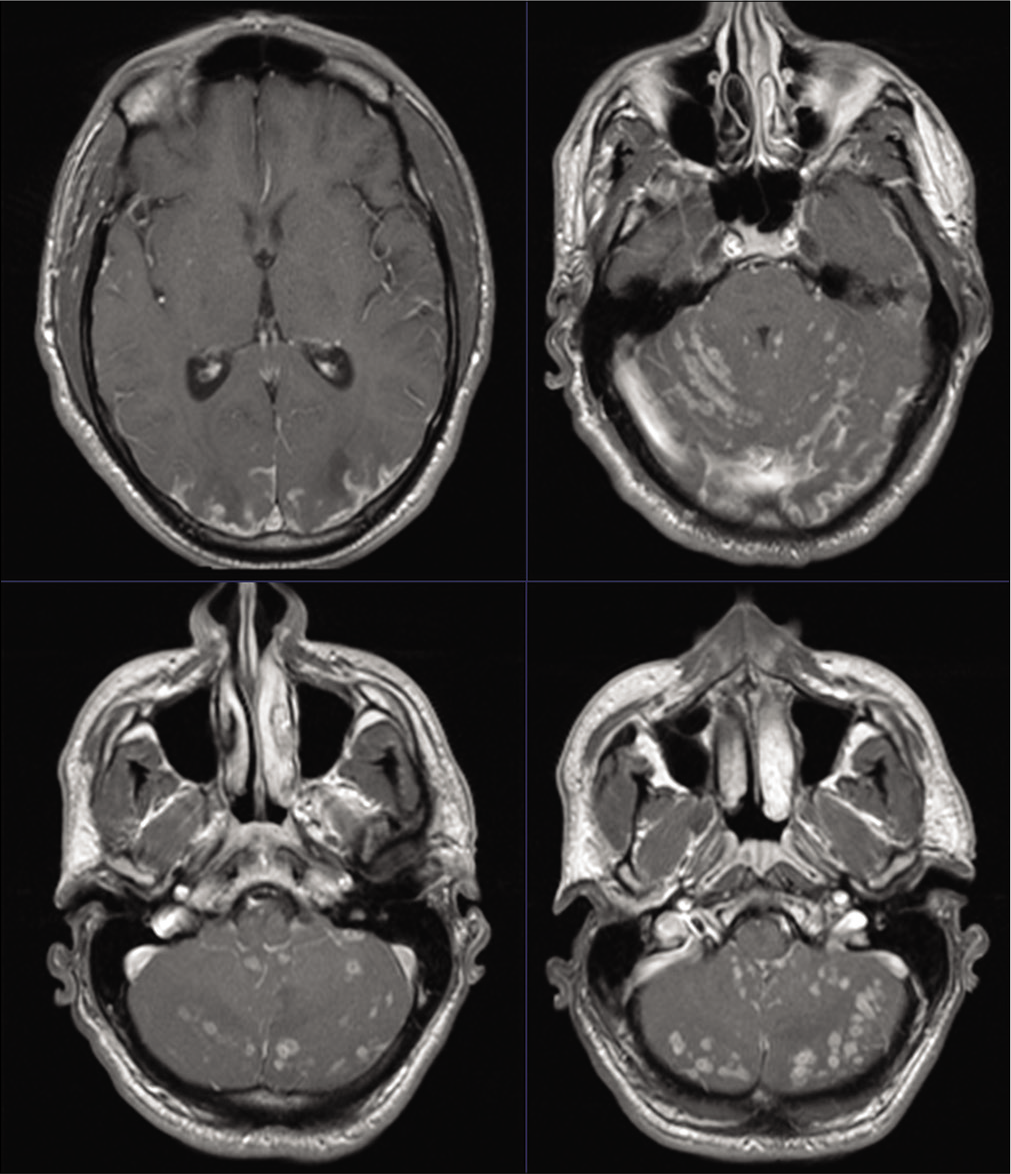
At 4-week follow-up, the patient complained of recurrent headaches and visual decline, nausea, and excessive sleepiness. His visual acuity was noted to stable at 20/70 on the left and improved at 20/50 on the right. Contrasted MRI that had been ordered as part of the routine follow-up demonstrated stable occipital FLAIR changes with residual occipital and cerebellar leptomeningeal enhancement, but more strikingly, the development of extensive tiny bilateral cerebellar enhancing lesions associated with edema, fourth ventricular compression, and early obstructive hydrocephalus [
He was admitted and underwent placement of a right frontal ventriculostomy with an opening pressure of 6 cm H2O and a xanthochromic appearance to the CSF [
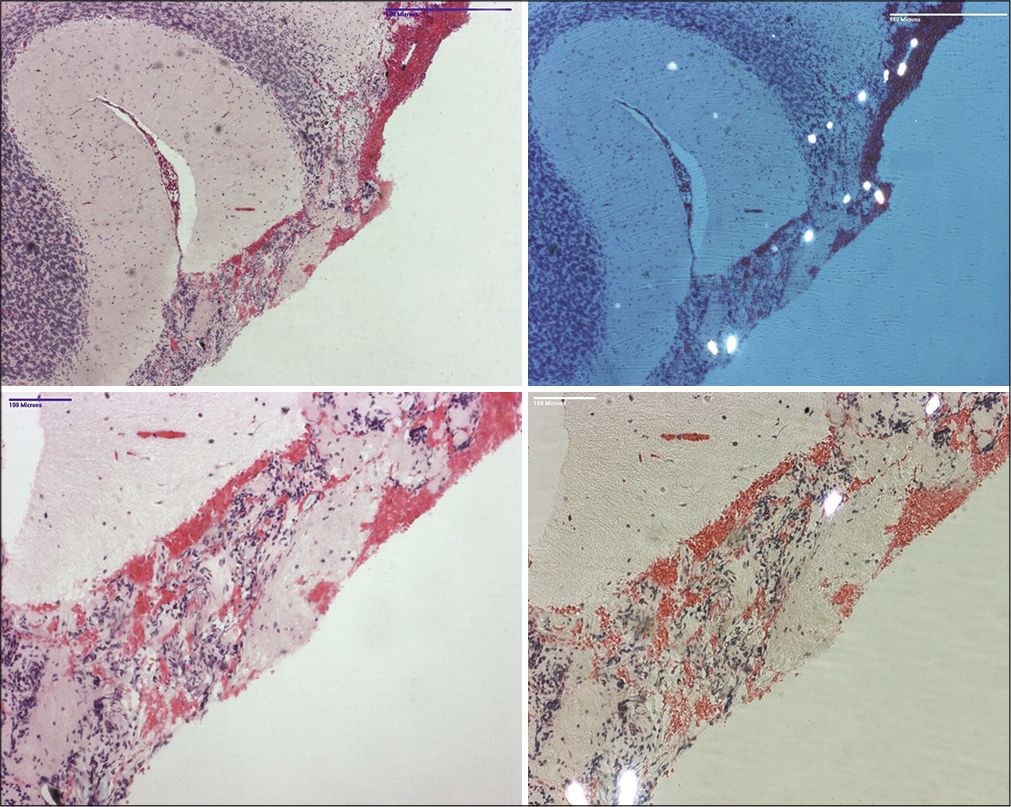
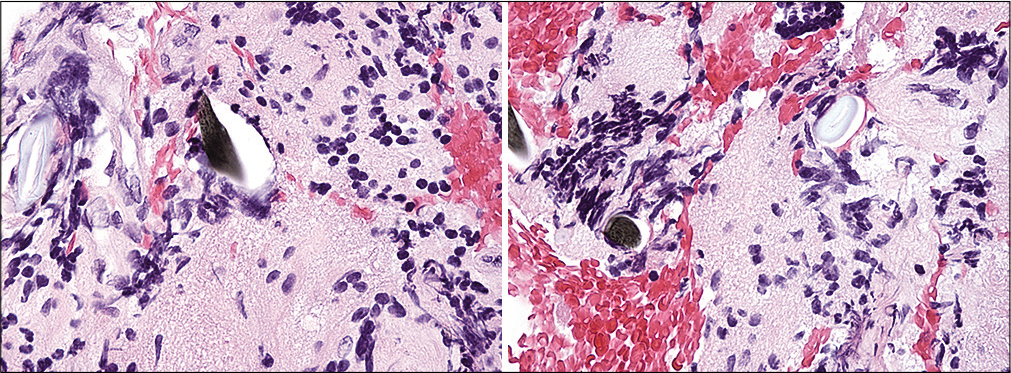
Final pathology from the posterior fossa biopsy revealed multiple elongated, diamond-shaped, avidly polarizable crystals in both the leptomeninges and cerebellar cortex [
MRI imaging 2 months after biopsy demonstrated significant improvement in occipital FLAIR changes and cerebellar enhancing lesions/edema. At last follow-up 2 years after his initial presentation, he had ongoing headaches and visual difficulties and imaging demonstrating residual bilateral occipital cortical encephalomalacia with resolution of the enhancement and edema [
DISCUSSION
Methamphetamines have long been implicated in the development of strokes in young people due to acute induction of hypertension as well as their chronic effects on blood pressure and their potential to cause vascular changes including vasculitis. Vasculitis is theorized to result from chronic direct toxicity to vessel walls and fibrinoid necrosis of the intima and media.[
The mechanism by which amphetamines contribute to RCVS or PRES likely extends beyond their ability to generate hypertension. In addition to injury and suppression of serotonergic and dopaminergic systems with chronic use, methamphetamines are thought to disrupt BBB function as well as the tight junction and structural proteins necessary for BBB integrity.[
The patient in this case did not have evidence of vasculitis on DSA or any of the characteristic changes expected on biopsy. The relatively normal vascular and cytoarchitecture on the pathology specimen speak against chronic changes related to methamphetamine use. Although he was never recorded to be hypertensive in the hospital, it is plausible that the patient’s admitted use of IV methamphetamines 3 weeks before his initial presentation resulted in a transient hypertensive episode and/or a direct BBB insult that set in motion the cascade of cerebral dysautoregulation that culminated in the development of PRES. The patient unfortunately did not undergo a new urine drug screen on his subsequent admission with the bizarre cerebellar lesions, but we hypothesize that in the interim between admissions, he may have used IV methamphetamines again. As mentioned previously, TTE did not demonstrate evidence of a cardiac septal defect. While some relatively large particles are known to traverse the pulmonary circulation, an undetected route of paradoxical embolism is another plausible mechanism of crystalline delivery. In the setting of nonfully recovered PRES and a leaky BBB, a “one-two punch” of large crystals depositing in the vulnerable leptomeningeal and cerebellar parenchymal vessels may have generated the observed clinical and radiographic syndrome. However, that does not explain the predilection for the previously radiographically uninvolved cerebellar hemispheres rather than the occipital lobes.
Systemically, pulmonary talcosis has been described in the setting of inhalational use of illicit substances but also through IV use of crushed substances cut or combined with talc powder and other similar agents, leading to characteristic perivascular deposition of birefringent crystals termed “talc granulomas.”[
CONCLUSION
The authors present what we believe to be the first documented case of CNS talcosis in a 35-year-old male IV methamphetamine abuser presenting with leptomeningeal enhancement, diffuse bilateral cerebellar enhancing lesions, vasogenic edema, and low pressure hydrocephalus while recovering from PRES. We hypothesize a mechanism of PRES resulting from aberrant autoregulation in the setting of a methamphetamine-related transient hypertensive episode and/or the direct effects of methamphetamine intoxication on the BBB, with the subsequent opportunistic deposition of talc crystals into susceptible CNS tissue. Further reports and studies are needed to better elucidate this rare pathology.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akins PT, Axelrod Y, Silverthorn JW, Guppy K, Banerjee A, Hawk MW. Management and outcomes of malignant posterior reversible encephalopathy syndrome. Clin Neurol Neurosurg. 2014. 125: 52-7
2. Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006. 60: 521-32
3. Castillo A, Payne JD, Nugent K. Posterior reversible leukoencephalopathy syndrome after kratom ingestion. Proc (Bayl Univ Med Cent). 2017. 30: 355-7
4. Dujella N, Dujella J. Patho-anatomic changes in a narcotic addict. Lijec Vjesn. 1991. 113: 415-7
5. Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D. The CARE guidelines: Consensus-based clinical case reporting guideline development. J Med Case Rep. 2013. 7: 223
6. Gözübüyük A, Ozpolat B, Ciçek AF, Caylak H, Yücel O, Kavaklı K. Comparison of side effects of oxytetracycline and talc pleurodesis: An experimental study. J Cardiothorac Surg. 2010. 5: 128
7. Griffith CC, Raval JS, Nichols L. Intravascular talcosis due to intravenous drug use is an underrecognized cause of pulmonary hypertension. Pulm Med. 2012. 2012: 617531
8. Iqbal A, Aggarwal B, Menon B, Kulshreshtha R. Talc granulomatosis mimicking sarcoidosis. Singapore Med J. 2008. 49: e168-70
9. Kresca LJ, Goldberg MF, Jampol LM. Talc emboli and retinal neovascularization in a drug abuser. Am J Ophthalmol. 1979. 87: 334-9
10. Kringsholm B, Christoffersen P. The nature and the occurrence of birefringent material in different organs in fatal drug addiction. Forensic Sci Int. 1987. 34: 53-62
11. Lappin JM, Darke S, Farrell M. Stroke and methamphetamine use in young adults: A review. J Neurol Neurosurg Psychiatry. 2017. 88: 1079-91
12. Minhaj FS, Schult RF, Dvorak P, Nacca N. Amphetamine and clonidine toxicity resulting in posterior reversible encephalopathy syndrome. Pediatr Emerg Care. 2019. p.
13. Mizutani T, Lewis RA, Gonatas NK. Medial medullary syndrome in a drug abuser. Arch Neurol. 1980. 37: 425-8
14. Murphy SB, Jackson WB, Pare JA. Talc retinopathy. Can J Ophthalmol. 1978. 13: 152-6
15. Northrop NA, Yamamoto BK. Methamphetamine effects on blood-brain barrier structure and function. Front Neurosci. 2015. 9: 69
16. Richards JR, Laurin EG.editors. Methamphetamine toxicity. Stat Pearls. Treasure Island, FL: Stat Pearls Publishing; 2020. p.
17. Siddiqui MF, Saleem S, Badireddi S. Pulmonary talcosis with intravenous drug abuse. Respir Care. 2013. 58: e126-8
18. Soliman MK, Sarwar S, Hanout M, Sadiq MA, Agarwal A, Gulati V. High-resolution adaptive optics findings in talc retinopathy. Int J Retina Vitreous. 2015. 1: 10
19. Tantikittichaikul S, Ruthirago D, Ali S, Claudio A, Kim J. Posterior reversible encephalopathy syndrome with multiple cerebellar mass-like lesions secondary to amphetamine (P4.403). Neurology. 2016. 86: P4.403
20. Tark BE, Messe SR, Balsucani C, Levine SR. Intracerebral hemorrhage associated with oral phenylephrine use: A case report and review of the literature. J Stroke Cerebrovasc Dis. 2014. 23: 2296-300
21. Werebe EC, Pazetti R, de Campos JR, Fernandez PP, Capelozzi VL, Jatene FB. Systemic distribution of talc after intrapleural administration in rats. Chest. 1999. 115: 190-3