- Department of Neurosurgery, Salford Royal NHS Foundation Trust, Salford, United Kingdom
- Department of Cellular Pathology, Salford Royal NHS Foundation Trust, Salford, United Kingdom
- Department of Clinical Genetics, Saint Mary’s Hospital, Manchester, United Kingdom
Correspondence Address:
Sayantan Bose, Department of Neurosurgery, Salford Royal NHS Foundation Trust, Salford, United Kingdom.
DOI:10.25259/SNI_315_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sayantan Bose1, James Balogun1, Daniel du Plessis2, Matthew Bailey1, Fiona Lalloo3, Omar Pathmanaban1. Cerebellopontine angle craniopharyngioma in familial adenomatous polyposis. 20-Sep-2024;15:340
How to cite this URL: Sayantan Bose1, James Balogun1, Daniel du Plessis2, Matthew Bailey1, Fiona Lalloo3, Omar Pathmanaban1. Cerebellopontine angle craniopharyngioma in familial adenomatous polyposis. 20-Sep-2024;15:340. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13106
Abstract
Background: Craniopharyngiomas are benign tumors arising in the sellar and suprasellar regions. Although ectopic tumors do occur, it is usually due to local spread or recurrent tumors. Purely ectopic cerebellopontine angle (CPA) or 4th ventricle tumors are extremely rare and have been found to be significantly associated with familial adenomatous polyposis (FAP), a genetic disorder.
Case Description: Only four cases of ectopic CPA craniopharyngioma associated with FAP have been reported to date. Here, we present the 5th case of ectopic CPA craniopharyngioma on a background of FAP. The previously described cases have been elaborated as well.
Conclusion: CPA tumor with a background of FAP should raise a differential diagnosis of craniopharyngioma, and similarly, a CPA primary ectopic craniopharyngioma may raise suspicion of underlying APC gene mutation.
Keywords: 4th ventricle craniopharyngioma, Cerebellopontine angle craniopharyngioma, Ectopic craniopharyngioma, Familial adenomatous polyposis, Gardner syndrome
INTRODUCTION
Craniopharyngiomas are benign brain tumors with a point prevalence of 0.5–2 cases/million population, and they constitute 2–5% of all intracranial tumors.[
We report a patient with FAP and an ectopic CPA craniopharyngioma, adding to the potential CPA craniopharyngioma predisposition in FAP.
CASE REPORT
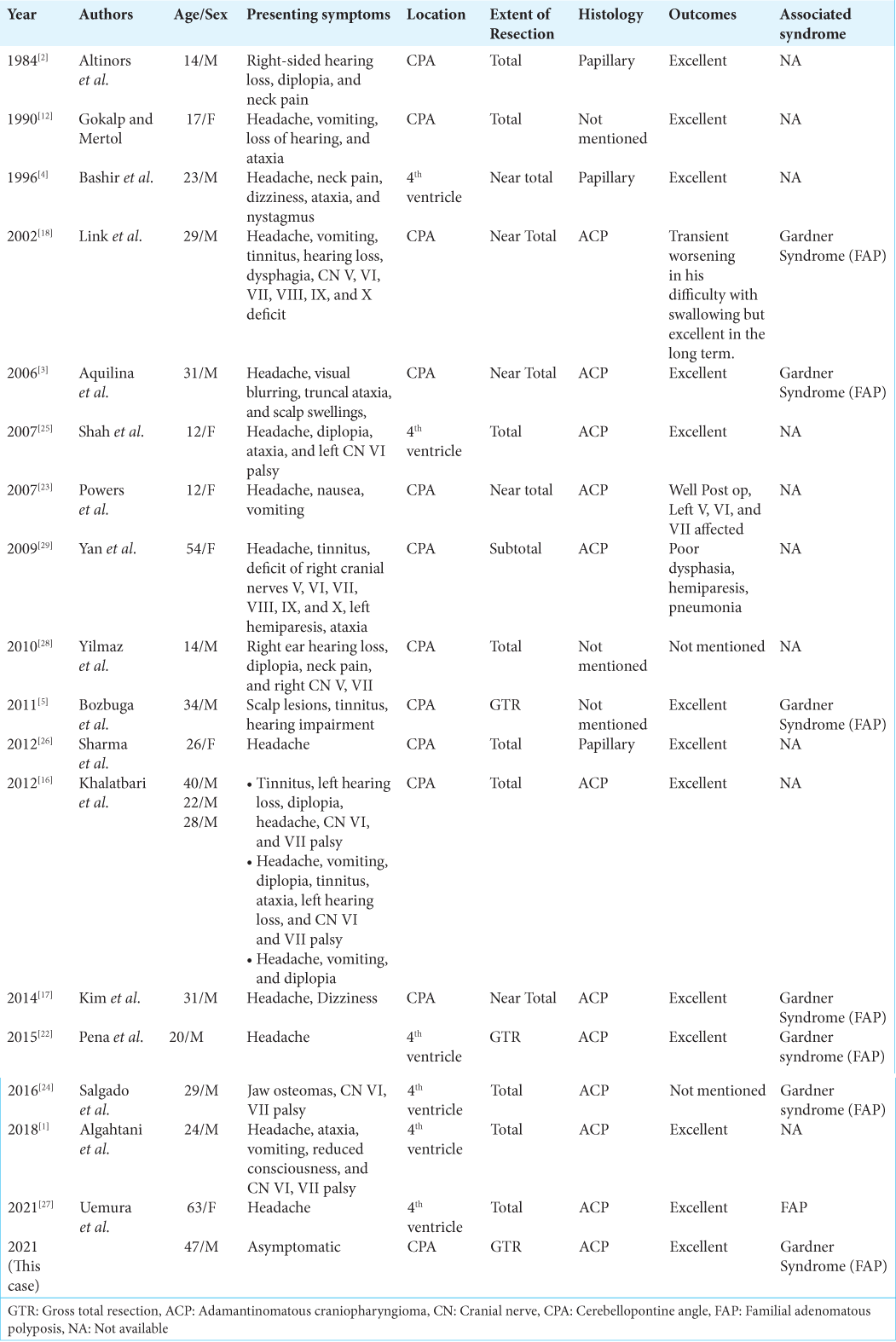
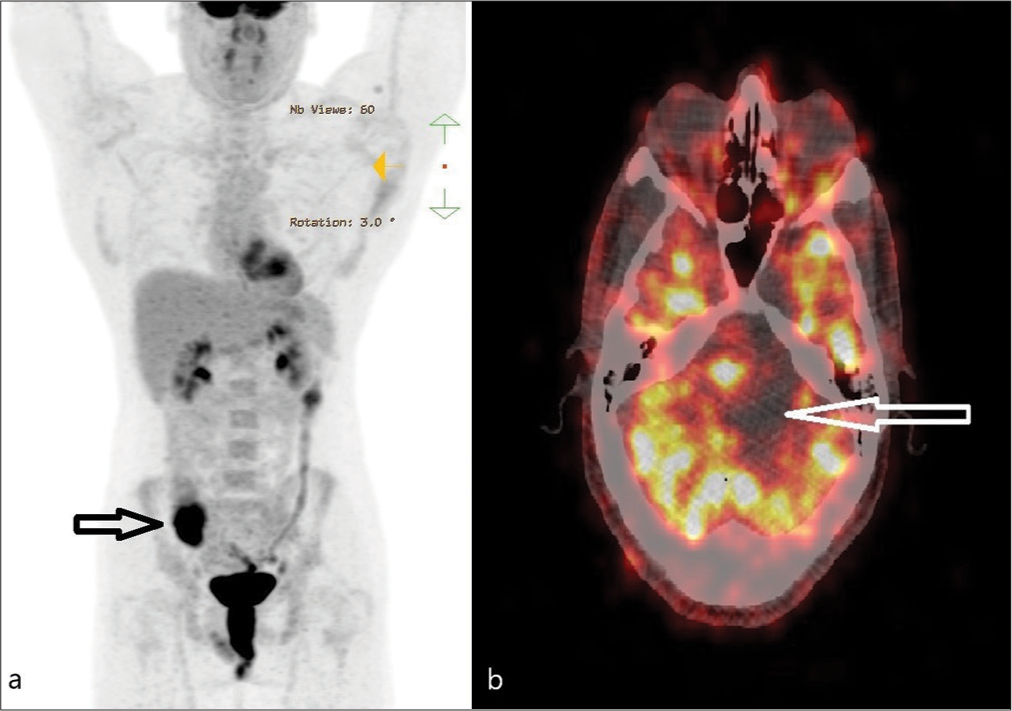
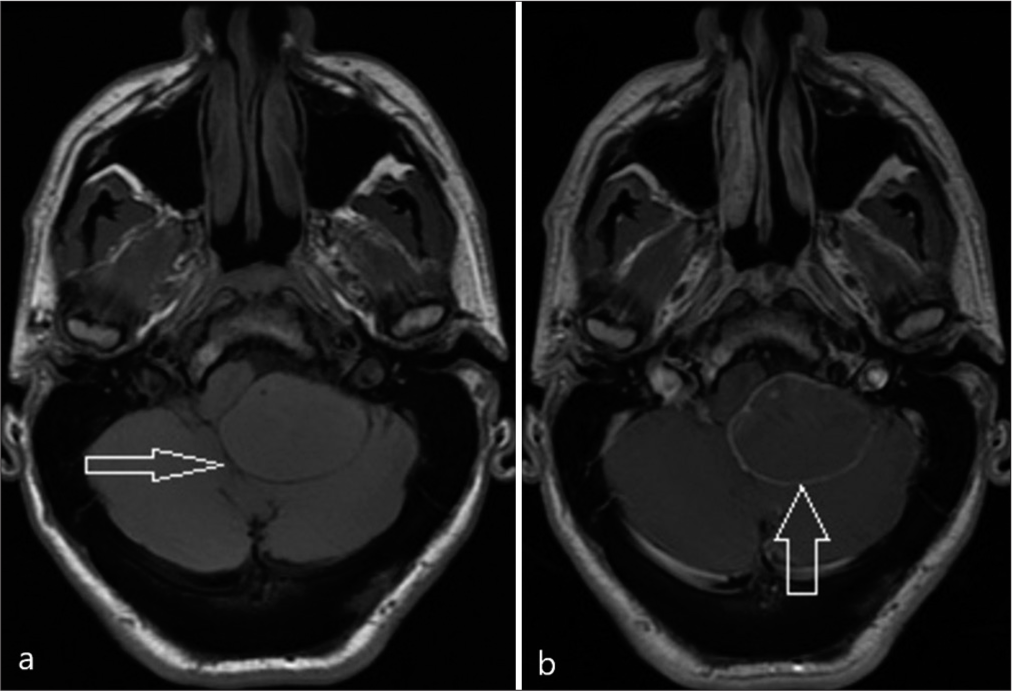
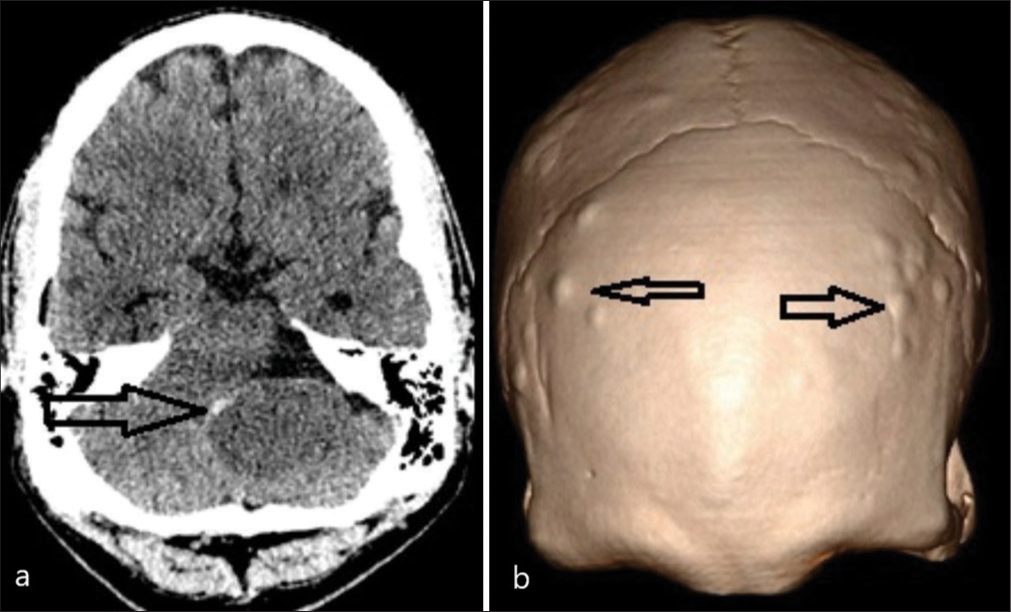
A 47-year-old male, known to have FAP with a confirmed pathogenic variant of APC on genetic testing aged 19, had declined recommended colorectal surveillance for a number of years. He presented with rectal bleeding, following which he was diagnosed with numerous colorectal polyps and a rectal adenocarcinoma. A screening positron emission tomography (PET) scan demonstrated areas of high FDG uptake in the colon and showed an area of photopenia in the posterior cranial fossa and a mass effect on the brain stem [
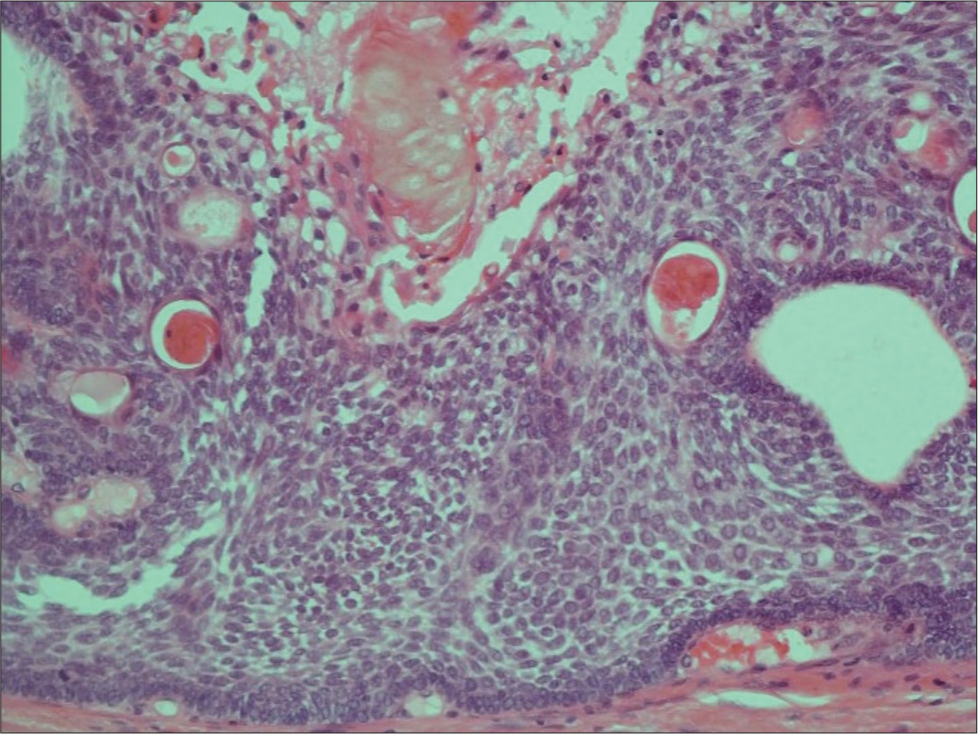
He underwent a retrosigmoid approach and gross total excision of the tumor, including the capsule, with intraoperative monitoring of cranial nerves. Adherent choroid plexus at Foramen of Luschka was observed and resected with tumor en bloc. The histology of the tumor was composed of stratified squamous epithelium showing architectural features of an adamantinomatous craniopharyngioma [
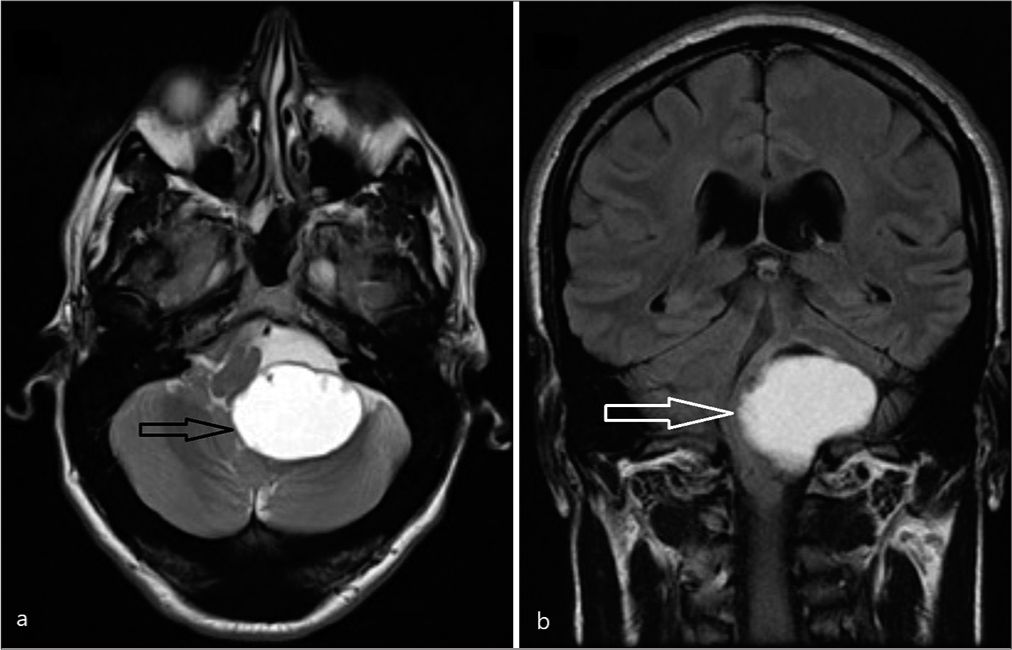
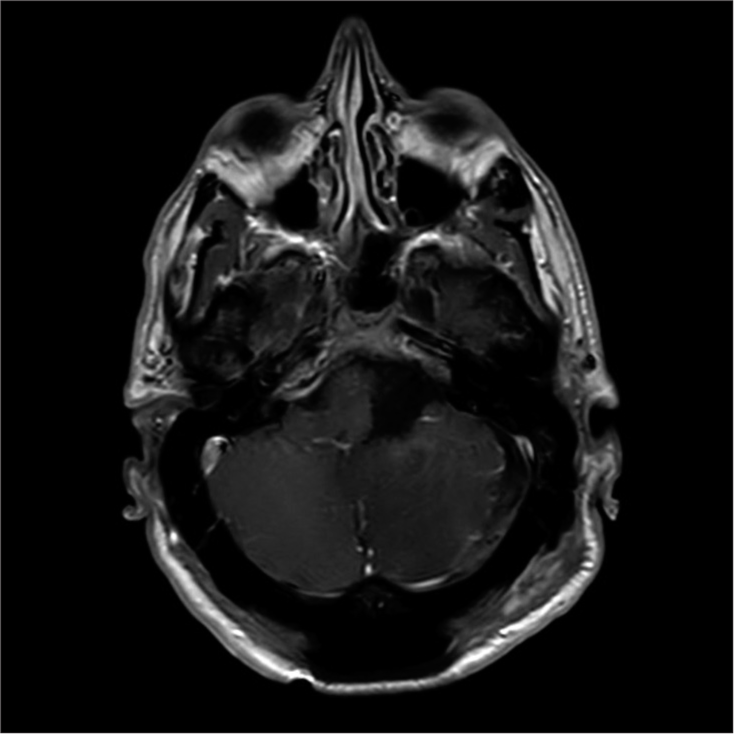
He remained neurologically intact postoperatively without any new deficits and has been subsequently reviewed in the clinic and continues to remain well. A postoperative MRI scan is shown in
DISCUSSION
Craniopharyngiomas located outside of the usual embryological locations of the Rathke’s pouch are not common, and CPA is particularly unusual. The presence of craniopharyngiomas at CPA has been theorized to include the migration and entrapment of squamous cells into the posterior fossa from the suprasellar location.[
Patients with craniopharyngioma can present with several clinical features, including endocrine dysfunction in the form of pan-hypopituitarism, selective hormone deficiency, or posterior pituitary/stalk dysfunction. With the increasing size of tumor, patients may present with features of raised intracranial pressure from obstructive hydrocephalus or with a disturbance in behaviour or mentation due to the effect on hypothalamus, frontal and temporal lobes.
As with any CPA tumor, there can be deficits of multiple cranial nerves. It is interesting to note that our patient was asymptomatic, while in earlier reports, the patients presented with features of raised intracranial pressure, cerebellar, and cranial nerve II, V, VI, VII, VIII, IX, and X symptoms. Screening protocol, including a PET-CT scan at the time of his diagnosis of colorectal cancer, incidentally identified the current lesion.
Nearly all adamantinomatous craniopharyngiomas are driven by somatic mutations in the CTNNB1 gene (encoding β-catenin) that affect β-catenin production.[
In FAP, the germline pathogenic variant in APC coupled with a second hit somatic mutation results in the lack of β-catenin degradation. In sporadic adamantinomatous craniopharyngioma, which is caused by somatic CTNNB1 mutation, there is production of mutated β-catenin. This is resistant to degradation by Axin complex or APC, thus causing accumulation of β-catenin in the cytoplasm and nucleus.[
Safe maximal surgical excision has been recommended in the treatment of craniopharyngiomas. Gross total excision has been noted to reduce the risk of recurrence.[
CONCLUSION
Craniopharyngioma in CPA is rare. We have described a report of a CPA craniopharyngioma in a man with FAP in addition to the few previously reported cases. Craniopharyngioma should be included as a differential diagnosis of CPA/4th ventricle tumors in FAP patients. Likewise, a CPA/4th ventricle craniopharyngioma should also raise suspicion of underlying FAP and may warrant investigation. Early diagnosis and safe maximal resection are the keys to a favorable outcome.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Algahtani AY, Algahtani HA, Jamjoom AB, Samkari AM, Marzuk YI. De novo craniopharyngioma of the fourth ventricle: Case report and review of literature. Asian J Neurosurg. 2018. 13: 62-5
2. Altinörs N, Senveli E, Erdoğan A, Arda N, Pak I. Craniopharyngioma of the cerebellopontine angle. Case report. J Neurosurg. 1984. 60: 842-44
3. Aquilina K, O’Brien DF, Farrell MA, Bolger C. Primary cerebellopontine angle craniopharyngioma in a patient with Gardner syndrome. Case report and review of the literature. J Neurosurg. 2006. 105: 330-3
4. Bashir EM, Lewis PD, Edwards MR. Posterior fast craniopharyngioma. Br J Neurosurg. 1996. 10: 613-5
5. Bozbuga M, Turan Suslu H, Hicdonmez T, Bayindir C. Primary cerebellopontine angle craniopharyngioma in a patient with Gardner syndrome. J Clin Neurosci. 2011. 18: 300-1
6. Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, DiasSantagata D, Thorner AR. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014. 46: 161-5
7. Carleton-Bland N, Kilday JP, Pathmanaban ON, Stivaros S, Kelsey A, Kamaly-Asl ID. Ventricular metastatic dissemination of a paediatric craniopharyngioma: Case report and literature review. Br J Neurosurg. 2017. 31: 474-7
8. Connolly ES, Winfree CJ, Carmel PW. Giant posterior fossa cystic craniopharyngiomas presenting with hearing loss. Report of three cases and review of the literature. Surg Neurol. 1997. 47: 291-9
9. Dandurand C, Sepehry AA, Asadi Lari MH, Akagami R, Gooderham P. Adult craniopharyngioma: Case series, systematic review, and meta-analysis. Neurosurgery. 2018. 83: 631-41
10. Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suárez C. Craniopharyngioma: A pathologic, clinical, and surgical review. Head Neck. 2012. 34: 1036-44
11. Gökalp HZ, Egemen N, Ildan F, Bacaci K. Craniopharyngioma of the posterior fossa. Neurosurgery. 1991. 29: 446-8
12. Gökalp HZ, Mertol T. Cerebellopontine angle craniopharyngioma. Neurochirurgia (Stuttg). 1990. 33: 20-1
13. Gooderham P, Akagami R, Asadi Lari MH, Sepehry AA, Dandurand C. Adult craniopharyngioma: Case series, systematic review, and meta-analysis. Neurosurgery. 2018. 83: 631-41
14. Gorelyshev A, Mazerkina N, Medvedeva O, Vasilyev E, Petrov V, Ryzhova M. Second-hit APC mutation in a familial adamantinomatous craniopharyngioma. Neuro Oncol. 2020. 22: 889-91
15. Haupt R, Magnani C, Pavanello M, Caruso S, Dama E, Garrè ML. Epidemiological aspects of craniopharyngioma. J Pediatr Endocrinol Metab. 2006. 19: 289-93
16. Khalatbari MR, Borghei-Razavi H, Samadian M, Moharamzad Y, Schick U. Isolated primary craniopharyngioma in the cerebellopontine angle. J Clin Neurosci. 2012. 19: 1516-9
17. Kim MS, Kim YS, Lee HK, Lee GJ, Choi CY, Lee CH. Primary intracranial ectopic craniopharyngioma in a patient with probable Gardner’s syndrome. J Neurosurg. 2014. 120: 337-41
18. Link MJ, Driscoll CL, Giannini C. Isolated, giant cerebellopontine angle craniopharyngioma in a patient with Gardner syndrome: Case report. Neurosurgery. 2002. 51: 221-5 discussion 225-6
19. MacDonald BT, Tamai K, He X. Wnt/b-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009. 17: 9-26
20. Novák Z, Chrastina J, Feitová V, Lzicarová E, Ríha I. Minimally invasive treatment of posterior fossa craniopharyngioma by means of navigated endoscopy. Minim Invasive Neurosurg. 2008. 51: 165-8
21. Ohmori K, Collins J, Fukushima T. Craniopharyngiomas in children. Pediatr Neurosurg. 2007. 43: 265-78
22. Pena AH, Chaudhry A, Seidman RJ, Peyster R, Bangiyev L. Ectopic craniopharyngioma of the fourth ventricle in a patient with Gardner syndrome. Clin Imaging. 2016. 40: 232-6
23. Powers CJ, New KC, McLendon RE, Friedman AH, Fuchs HE. Cerebellopontine angle craniopharyngioma: Case report and literature review. Pediatr Neurosurg. 2007. 43: 158-63
24. Salgado JA, de Mesa FG, Ledezma JJ, Méndez ML, Sansinenea IP, de Lope-Llorca AR. Craneofaringioma ectópico y síndrome de Gardner: A propósito de un caso y revisión de la literatura. Neurocirugía. 2017. 28: 97-101 (In Spanish)
25. Shah GB, Bhaduri AS, Misra BK. Ectopic craniopharyngioma of the fourth ventricle: Case report. Surg Neurol. 2007. 68: 96-8
26. Sharma M, Mally R, Velho V, Hrushikesh K. Primary isolated cerebellopontine angle papillary craniopharyngioma. Neurol India. 2012. 60: 438-9
27. Uemura H, Tanji M, Natsuhara H, Takeuchi Y, Hoki M, Sugimoto A. The association of ectopic craniopharyngioma in the fourth ventricle with familial adenomatous polyposis: Illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE21572
28. Yilmaz C, Altinors N, Sonmez E, Gulsen S, Caner H. Rare lesions of the cerebellopontine angle. Turk Neurosurg. 2010. 20: 390-7
29. Yan Y, Tang WY, Yang G, Zhong D. Isolated cerebellopontine angle craniopharyngioma. J Clin Neurosci. 2009. 16: 1655-7