- Department of Neurosurgery, Matsunami General Hospital, Kasamatsu, Japan.
Correspondence Address:
Nozomi Sasaki, Department of Neurosurgery, Matsunami General Hospital, Kasamatsu, Japan.
DOI:10.25259/SNI_108_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nozomi Sasaki, Taku Hiramatsu, Yoshihito Hasegawa, Motoshi Sawada. Cerebral hemorrhage due to intracranial venous reflux associated with left brachiocephalic vein occlusion in a hemodialysis patient. 03-Mar-2023;14:79
How to cite this URL: Nozomi Sasaki, Taku Hiramatsu, Yoshihito Hasegawa, Motoshi Sawada. Cerebral hemorrhage due to intracranial venous reflux associated with left brachiocephalic vein occlusion in a hemodialysis patient. 03-Mar-2023;14:79. Available from: https://surgicalneurologyint.com/surgicalint-articles/12179/
Abstract
Background: Although central venous occlusion is sometimes seen in hemodialysis (HD) patients, neurological symptoms due to intracranial venous reflux (IVR) are extremely rare.
Case Description: We present a case of a 73-year-old woman with cerebral hemorrhage due to IVR associated with HD. She presented with lightheadedness and alexia, and was diagnosed with subcortical hemorrhage. Venography through the arteriovenous graft showed occlusion of the left brachiocephalic vein (BCV) and IVR through the internal jugular vein (IJV). It is extremely rare that IVR occurs and causes neurological symptoms. This is because that there is the presence of a valve in the IJV and the communication between the right and left veins through the anterior jugular vein and thyroid vein. Percutaneous transluminal angioplasty for the left obstructive BCV was performed, but the obstructive lesion was only slightly improved. Hence, shunt ligation was performed.
Conclusion: When IVR is found in HD patients, central veins should be confirmed. Early diagnosis and therapeutic intervention are desirable when neurological symptoms are present.
Keywords: Brachiocephalic vein, Central vein stenosis, Hemodialysis, Intracranial venous reflux
INTRODUCTION
Although central venous stenosis (CVS) or occlusion is sometimes seen in hemodialysis (HD) patients, neurological symptoms due to intracranial venous reflux (IVR) are very rare. Chronic intracranial venous stasis can cause venous infarction and hemorrhage, so immediate intervention is required when IVR is suspected. We present a case of cerebral hemorrhage due to IVR associated with the left brachiocephalic vein (BCV) occlusion in a HD patient.
CASE PRESENTATION
A 73-year-old woman suffering from end-stage renal disease due to glomerulonephritis and receiving HD through an arteriovenous fistula (AVF) in the left upper arm for 4 years presented with lightheadedness and alexia. Computed tomography showed a high-density area in the left occipital lobe, which was hypointense on T2 star weighted image of magnetic resonance imaging (MRI), and was diagnosed with subcortical hemorrhage [
Figure 1:
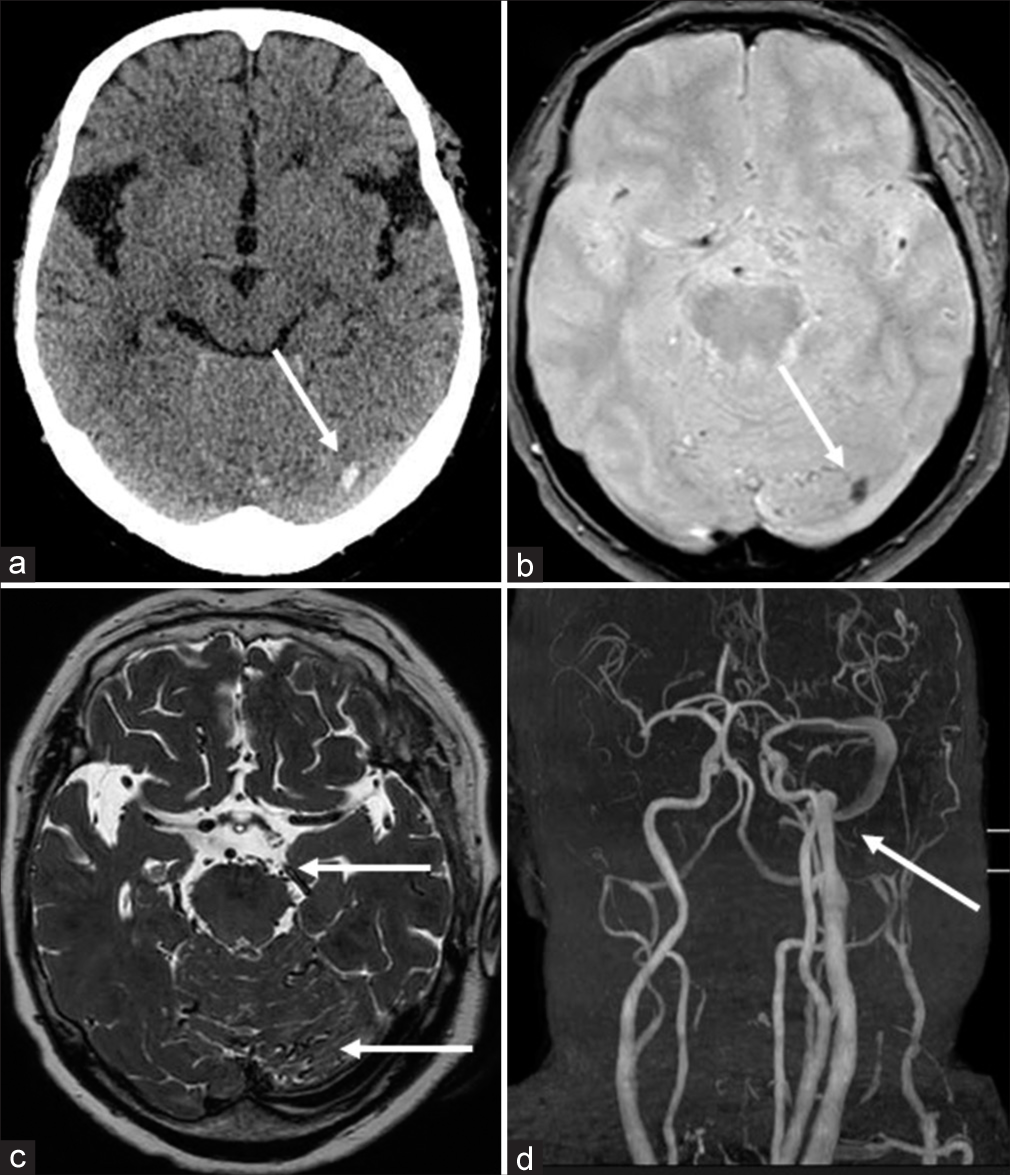
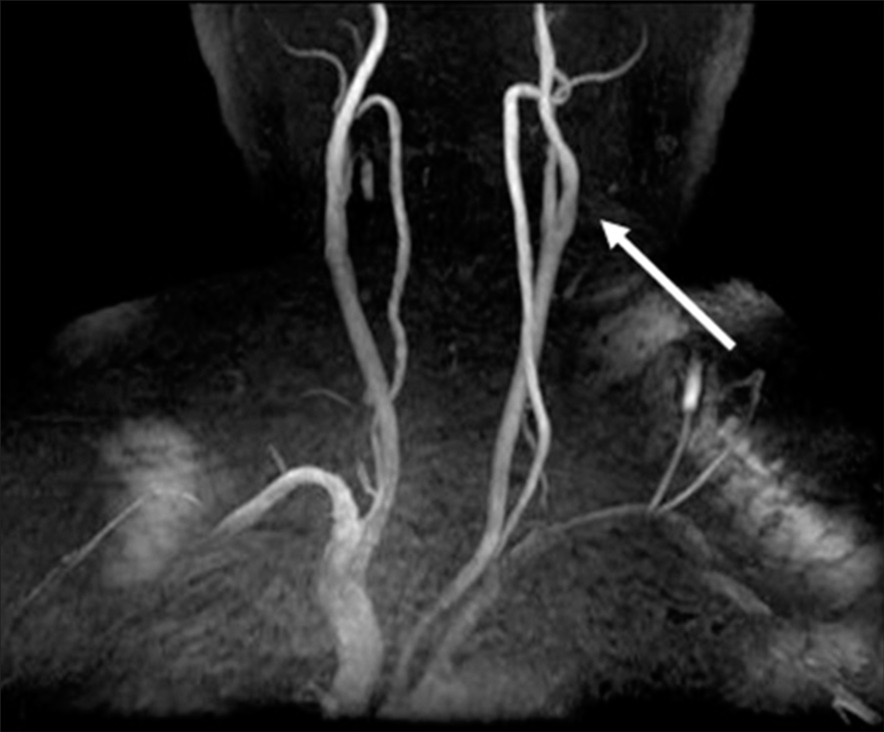
Imaging findings on admission (a and b) CT shows a slight high-density area in the left occipital lobe (arrow), which is hypointense on T2 star weighted image of MRI (arrow). (c) T2-weighted image of MRI shows flow voids around the cerebellum and brainstem (arrows). (d) MRA clearly visualizes the IJV and sigmoid sinus (arrow), and venous reflux into the anterior condylar vein and cavernous sinus can also be confirmed. CT: Computed tomography, MRI: Magnetic resonance imaging, MRA: Magnetic resonance angiography, IJV: Internal jugular vein.
Figure 2:
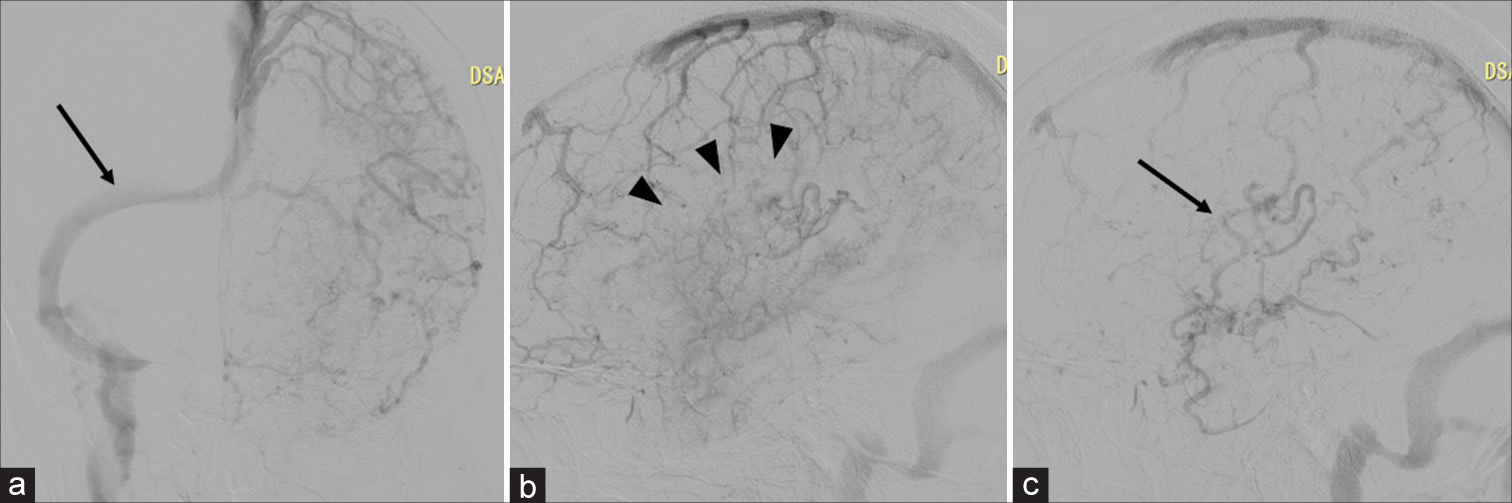
Left common carotid angiography (a) Venous perfusion of the left cerebral hemisphere uses the right transverse sinus (arrow), and the left transverse sinus is not visualized. (b and c) The venous blood flow of the left cerebral hemisphere reflux into cortical veins (arrow). Venous stasis and venous perfusion failure are detected (arrowheads).
Figure 3:
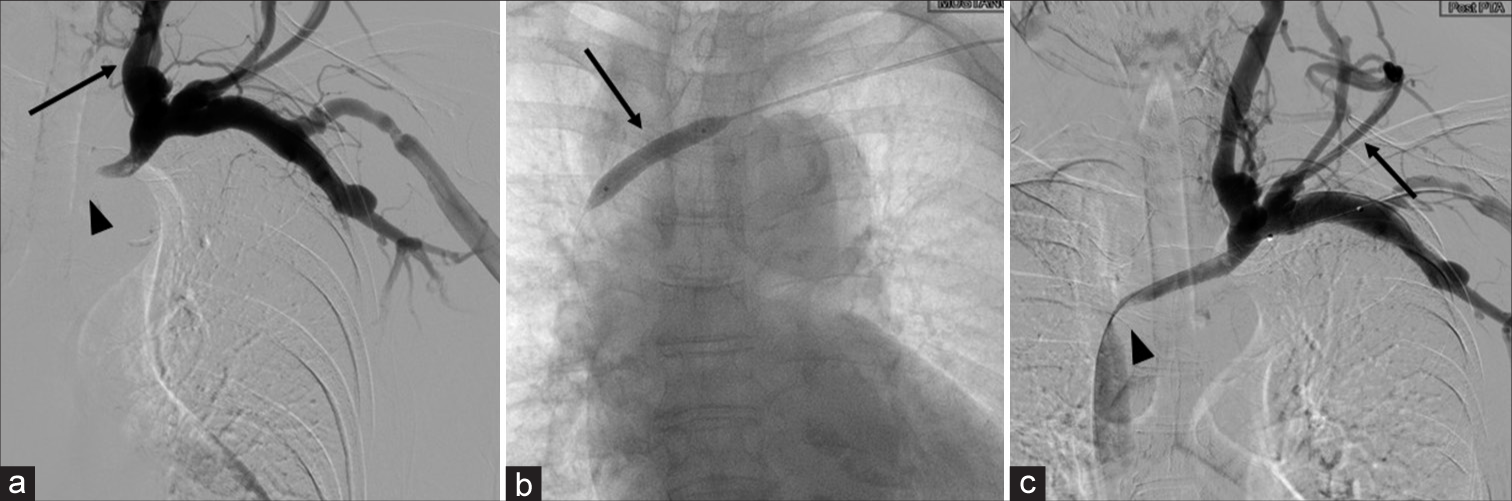
Venography through the arteriovenous graft (a) The left BCV is occluded (arrowhead) and veins flow back into the IJV (arrow), (b) Undergoing PTA for the left obstructive BCV (arrow), (c) The stenosis of the left BCV is only slightly improved (arrowhead). But reflux into the IJV remains and the ectasia of the subcutaneous veins in the left upper arm and neck is observed (arrow). BCV: Brachiocephalic vein, IJV: Internal jugular vein, PTA: Percutaneous transluminal angioplasty.
Percutaneous transluminal angioplasty (PTA) for the left obstructive BCV was performed, but the obstructive lesion was only slightly improved [
DISCUSSION
It is said that HD patients sometimes develop CVS. The causes of CVS are thought to be intimal hyperplasia due to chronic elevation of venous pressure, vasoconstriction, platelet aggregation, changes of shear stress and blood flow velocity, and intimal thickening due to oxidative stress.[
To the best of our knowledge, 23 cases of neurological symptoms due to IVR have been reported, but only three of them presented with cerebral hemorrhage.[
Although it is relatively easy to detect abnormal findings from the visualization of the venous sinuses by MRA, it is difficult to distinguish them from dAVF by MRI alone.
Angiography is the gold standard for definitive diagnosis. In cerebral angiography, it is important to confirm the denial of dural shunt in the arterial phase, venous stasis in the venous phase, and the use of alternative routes such as the right transverse sinus. Furthermore, it is also necessary to confirm the stenosis or occlusion of central vein, findings of IVR, and the development of collateral circulation, by venography through the arteriovenous graft. When there are abnormal findings of the venous sinuses in HD patients on head MRA, it is important to suspect CVS and perform not only cerebral angiography but also venography through the arteriovenous graft.
According to the National Kidney Foundation Guideline, PTA is the first choice for the treatment of symptomatic CVS in HD patients, and shunt ligation is the second choice.[
CONCLUSION
When IVR is found in HD patients, central veins should be confirmed. Early diagnosis and therapeutic intervention are desirable when neurological symptoms are present.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Agarwal AK. Central vein stenosis. Am J Kidney Dis. 2013. 61: 1001-15
2. Haruma J, Escalard S, Smajda S, Piotin M. Left temporal hemorrhage caused by cerebral venous reflux of a brachiobrachial hemodialysis fistula. Neuroratiology. 2020. 62: 1341-4
3. Herzig DW, Stemer AB, Bell RS, Liu AH, Armonda RA, Bank WO. Neurological sequelae from brachiocephalic vein stenosis. J Neurosurg. 2013. 118: 1058-62
4. Jang J, Kim BS, Kim BY, Choi HS, Jung SL, Ahn KJ. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuororadiology. 2013. 55: 1205-11
5. Kim CH, Kang J, Choi DS, Park JH. Intracranial venous reflux caused by occlusion of the brachiocephalic vein mimicking dural arteriovenous fistula. World Neurosurg. 2018. 120: 438-41
6. Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020. 75: S1-164
7. Zambrano FC, Palacio CM, Garbugino S, Gonzalez FM, Biolcati MB, Saucedo MA. Central venous reflux, a rare cause of neurological manifestations in hemodialysis patients: A case report and literature review. Neurointervention. 2022. 17: 58-64