- Department of Internal Medicine, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii, United States.
- Department of Neurological Surgery, John A. Burns School of Medicine, Honolulu, Hawaii, United States.
- Department of Medicine, John A. Burns School of Medicine, Honolulu, Hawaii, United States.
- Neurosurgery, John A. Burns School of Medicine, Honolulu, Hawaii, United States.
Correspondence Address:
Parthav Shah, Department of Internal Medicine, University of Hawaii John A. Burns School of Medicine, Honolulu, Hawaii, United States.
DOI:10.25259/SNI_532_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Torrey Czech1, Parthav Shah1, Gunnar Lee2, Gina Watanabe3, Christian Ogasawara3, Thomas Noh4. Cerebral toxoplasmosis in a patient with combined variable immunodeficiency. 12-Aug-2022;13:354
How to cite this URL: Torrey Czech1, Parthav Shah1, Gunnar Lee2, Gina Watanabe3, Christian Ogasawara3, Thomas Noh4. Cerebral toxoplasmosis in a patient with combined variable immunodeficiency. 12-Aug-2022;13:354. Available from: https://surgicalneurologyint.com/surgicalint-articles/11792/
Abstract
Background: Cerebral toxoplasmosis is an opportunistic infection in patients but has rarely been described in the setting of compromised humoral immunodeficiency. Prompt diagnosis and treatment of the infection is critical in the care of these patients. Medical management is the mainstay of treatment of the infection. There have been very few reports of surgical management of cerebral toxoplasmosis.
Case Description: We describe the case of a 40-year-old male who presented with headache, memory deficits, weight loss, and left-sided weakness in the setting of a known but undiagnosed brain lesion identified 1 month prior. Imaging demonstrated a right basal ganglia lesion which was initially presumed to be malignancy. On further workup including a positive serum test and biopsy including polymerase chain reaction analysis, diagnosis was confirmed as toxoplasmosis. On further investigation, he was found to have deficiencies in immunoglobulins consistent with common variable immunodeficiency (CVID). The patient underwent craniotomy with surgical debulking as repeat imaging showed increased size of mass with new satellite lesions and worsening hydrocephalus.
Conclusion: Cerebral toxoplasmosis is an important differential to consider in cases of intracerebral lesions and should not necessarily be excluded in the absence of compromised cellular immunity. In cases where there is no immunocompromised state and malignancy cannot immediately be established, CVID should be considered as an etiology. Due to the subtlety of CVID diagnosis, careful attention should be paid to history taking and workup for CVID should be considered as soon as possible. Surgical removal of these lesions in conjunction with medications is an effective treatment option.
Keywords: Cerebral toxoplasmosis, Common variable immunodeficiency, Craniotomy, Surgical debulking
INTRODUCTION
Cerebral toxoplasmosis is a life-threatening infection caused by reactivation of the protozoan parasite Toxoplasma gondii in primarily immunocompromised individuals.[
Common variable immunodeficiency (CVID) is an often underdiagnosed primary immune deficiency characterized by impaired B-cell differentiation, leading to decreased serum levels of immunoglobulin (Ig)G, IgA, and/or IgM.[
CASE REPORT
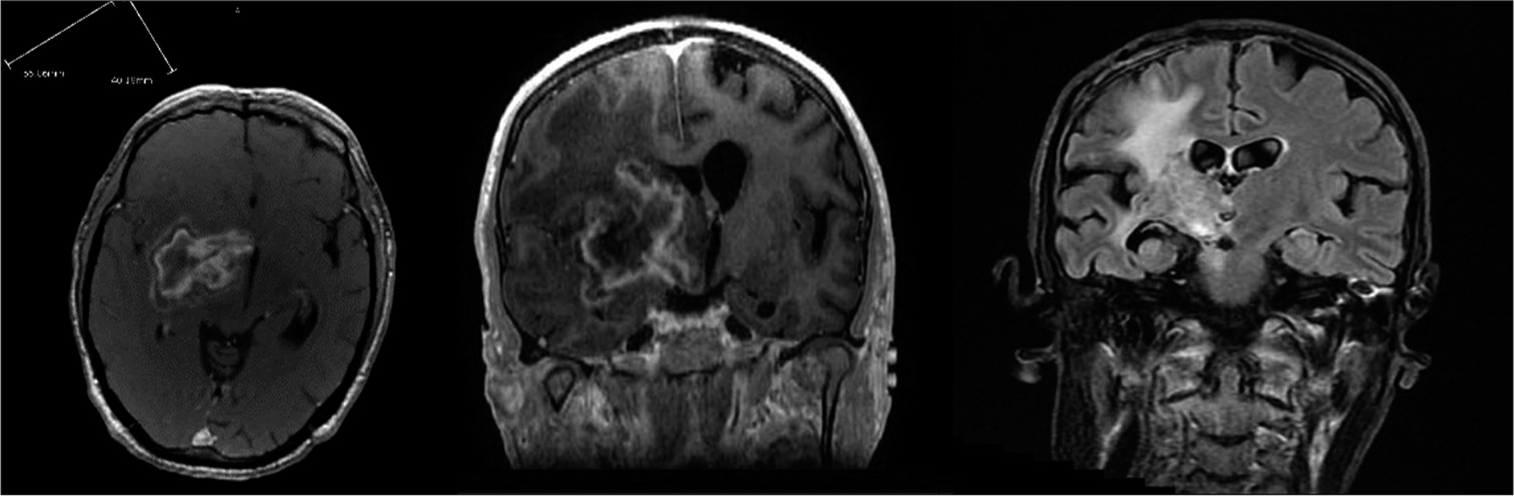
A 40-year-old Caucasian male with a 2-month history of headache, memory problems, weight loss, chronic diarrhea, and arthralgias, presented to an outside hospital with new-onset significant left-sided weakness impacting his ability to perform his activities of daily living. Medical history was remarkable for recurrent sinus infections and recurrent otitis media. He had no family history of central nervous system diseases or immunologic disease. CT head demonstrated an ill-defined mass in the right gangliocapsular region and midbrain and approximately 10 mm of leftward subfalcine herniation. MRI demonstrated a 4.4 × 2.4 × 4.5 cm right basal ganglia lesion with extension into midbrain, peripheral enhancement with extensive surrounding edema, and 7 mm midline shift [
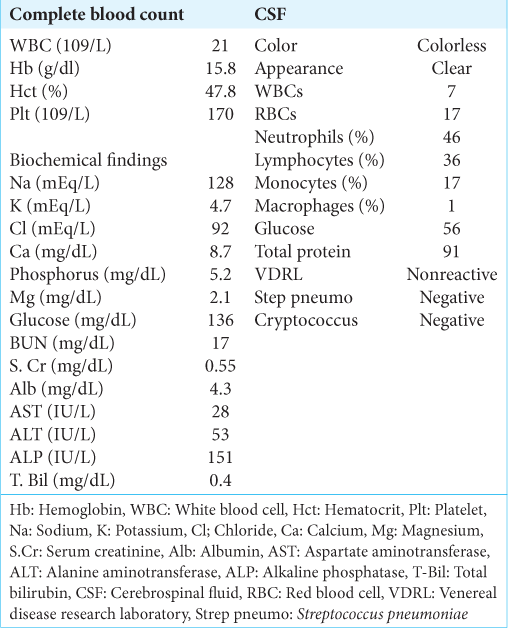
He then presented to our hospital with worsening weakness. On examination, he had 0/5 strength on his left upper extremity, left side facial droop, midline palatal elevation, midline tongue protrusion, and right-sided ptosis. Laboratory tests on admission are summarized in
One week later, despite being on antibiotics and antifungals, his neurological examination deteriorated and he became more obtunded and less responsive. A repeat MRI showed an increase in size of mass with new satellite lesions and hydrocephalus. The decision was made to perform a craniotomy for excision of enlarging mass to debulk and obtain tissue specimens for diagnosis.
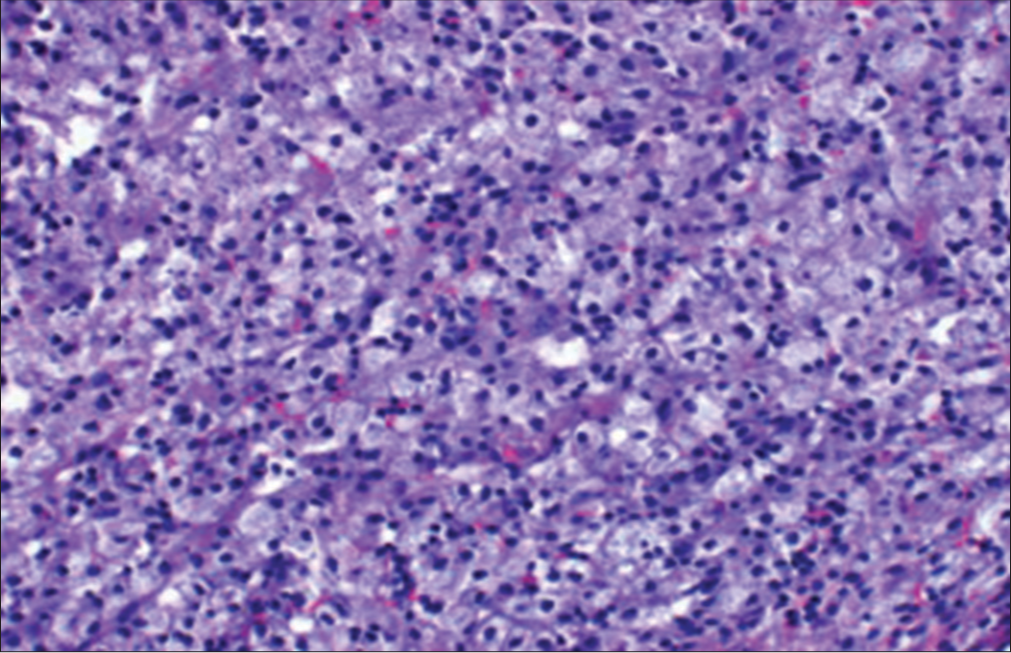
During the operation, there was a tough rind that was encountered and dissected around. Initial specimens did not indicate any obvious tumor, and thus, a pericapsular dissection was continued until most of the mass was removed with a small rind left around the midbrain. Specimen was sent for pathology, culture, and analysis. Postoperatively, he was intubated but opened his eyes to voice and followed commands on his right side. He was extubated on POD 7 and was AO × 3 with dense left hemiplegia.
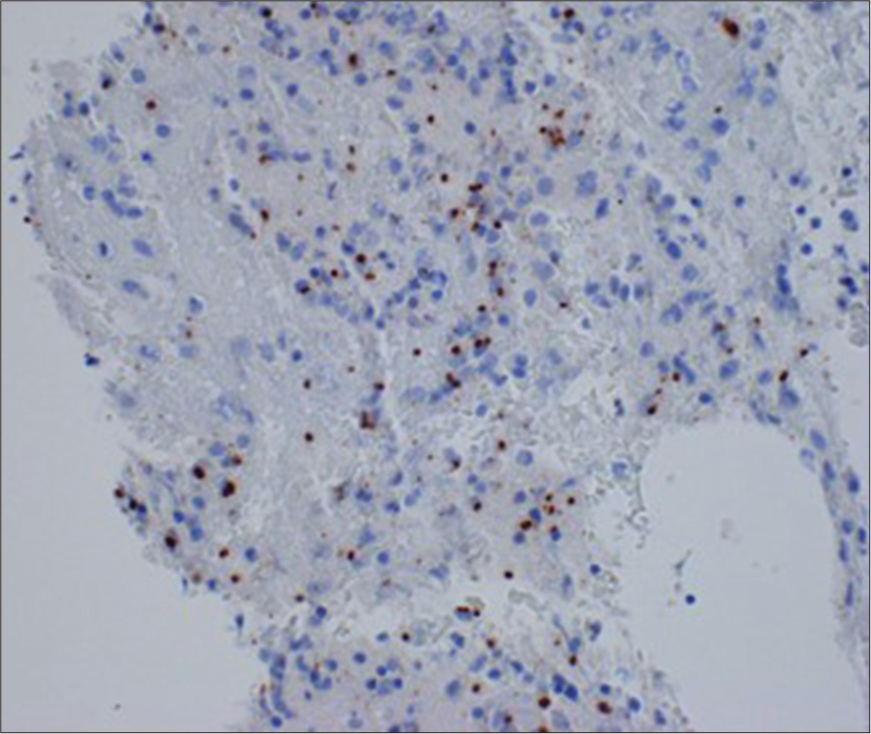
Polymerase chain reaction analysis of the mass was positive for toxoplasmosis gondii [
DISCUSSION
Cerebral toxoplasmosis is caused by T. gondii, a ubiquitous obligate intracellular parasite.[
Cerebral toxoplasmosis is an opportunistic disease seen in patients with a compromised cellular immunity. Interferon-dependent, CD8+ T cell-mediated immunity is the mainstay of resistance against cerebral toxoplasmosis.[
One rare component of this case was the presentation of cerebral toxoplasmosis as a large solitary lesion, initially thought to be more characteristic of a primary brain malignancy. Given the solitary lesion and presence of enlarged lymph nodes, a presumptive diagnosis of lymphoma was made which contributed to a delay in diagnosis and treatment of the toxoplasmosis. Solitary lesions in an immunocompromised patient are more likely associated with either primary central nervous system (CNS) lymphoma in higher-income countries or tuberculomas in the developing countries.[
Early presumptive diagnosis and empirical treatment are crucial to the management of cerebral toxoplasmosis. However, the absence of an existing immunosuppressive diagnosis as well as atypical radiographic features may delay appropriate treatment including surgery. The previous studies have shown that patients on medical management experience CNS response in 5 days and about 95% of patients show radiographic improvement by day 14 of treatment.[
CONCLUSION
We described a patient of cerebral toxoplasmosis with CVID who underwent craniotomy with surgical debulking. In our patient, the patient was initially diagnosed with cerebral toxoplasmosis before being diagnosed with CVID. Although cerebral toxoplasmosis is often associated with HIV, the diagnosis should be included in the differential for any individual with suspected or confirmed immunodeficiency. It should be suspected in patients with humoral immunodeficiency along with cell-mediated immunodeficiency. Furthermore, clinicians should look for undiagnosed immunodeficiency in patients with confirmed diagnosis of cerebral toxoplasmosis. Accurate diagnosis can be challenging especially when in cases, where the immunodeficiency is undiagnosed and radiologic imaging studies demonstrate a solitary lesion as it was seen in our case, which can lead to a delay in treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abbasi Fard S, Khajeh A, Khosravi A, Mirshekar A, Masoumi S, Tabasi F. Fulminant and diffuse cerebral toxoplasmosis as the first manifestation of HIV infection: A case presentation and review of the literature. Am J Case Rep. 2020. 21: e919624
2. Adamo MA, Deshaies EM. Emergency decompressive craniectomy for fulminating infectious encephalitis. J Neurosurg. 2008. 108: 174-6
3. Agrawal D, Hussain N. Decompressive craniectomy in cerebral toxoplasmosis. Eur J Clin Microbiol Infect Dis. 2005. 24: 772-3
4. Basavaprabhu A, Soundarya M, Deepak M, Satish R. CNS toxoplasmosis presenting with obstructive hydrocephalus in patients of retroviral disease--a case series. Med J Malaysia. 2012. 67: 214-6
5. Basavaraju A. Toxoplasmosis in HIV infection: An overview. Trop Parasitol. 2016. 6: 129-35
6. Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg. 1990. 73: 720-4
7. Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012. 7: 1-17
8. Eggers C, Vortmeyer A, Emskötter T. Cerebral toxoplasmosis in a patient with the acquired immunodeficiency syndrome presenting as obstructive hydrocephalus. Clin Neuropathol. 1995. 14: 51-4
9. Erdag N, Bhorade RM, Alberico RA, Yousuf N, Patel MR. Primary lymphoma of the central nervous system: Typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. 2001. 176: 1319-26
10. Ernst TM, Chang L, Witt MD, Aronow HA, Cornford ME, Walot I. Cerebral toxoplasmosis and lymphoma in AIDS: Perfusion MR imaging experience in 13 patients. Radiology. 1998. 208: 663-9
11. Hofmann A, Zaharatos G, Miller M. Case report and review of the literature: Toxoplasma gondii encephalitis in a 40-year-old woman with common variable immunodeficiency and a new diagnosis of large granular lymphocytic leukemia. Can J Infect Dis Med Microbiol. 2008. 19: 309-10
12. Holtkamp M, Okuducu AF, Klingebiel R, Ploner CJ. Cerebral toxoplasmosis in a patient with common variable immunodeficiency. Neurology. 2004. 63: 2192-3
13. Jones JL, Kruszon-Moran D, Elder S, Rivera HN, Press C, Montoya JG.editors. Toxoplasma gondii Infection in the United States, 2011-2014. Am J Trop Med Hyg. 2018. 98: 551-7
14. Kang H, Remington JS, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000. 164: 2629-34
15. Lee AM, Bai HX, Zou Y, Qiu D, Zhou J, Martinez-Lage Alvarez M. Safety and diagnostic value of brain biopsy in HIV patients: A case series and meta-analysis of 1209 patients. J Neurol Neurosurg Psychiatry. 2016. 87: 722-33
16. Luft BJ, Hafner R, Korzun AH, Leport C, Antoniskis D, Bosler EM. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 study team. N Engl J Med. 1993. 329: 995-1000
17. Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992. 15: 211-22
18. Malphettes M, Gérard L, Carmagnat M, Mouillot G, Vince N, Boutboul D. Late-onset combined immune deficiency: A subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis. 2009. 49: 1329-38
19. Manwani N, Ravikumar K, Viswanathan V, Rao SM, Mahadevan A. Acquired toxoplasmosis presenting with a brainstem granuloma in an immunocompetent adolescent. Indian Pediatr. 2016. 53: 159-61
20. Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. Qjm. 2004. 97: 413-21
21. Mrusek S, Marx A, Kummerle-Deschner J, Tzaribachev N, Enders A, Riede UN. Development of granulomatous common variable immunodeficiency subsequent to infection with Toxoplasma gondii. Clin Exp Immunol. 2004. 137: 578-83
22. Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992. 327: 1643-8
23. Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000. 68: 1026-33
24. Sell M, Klingebiel R, Di Iorio G, Sampaolo S. Primary cerebral toxoplasmosis: A rare case of ventriculitis and hydrocephalus in AIDS. Clin Neuropathol. 2005. 24: 106-11
25. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988. 240: 516-8
26. Tam JS, Routes JM. Common variable immunodeficiency. Am J Rhinol Allergy. 2013. 27: 260-5
27. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: From animals to humans. Int J Parasitol. 2000. 30: 1217-58
28. Vidal JE. HIV-related cerebral toxoplasmosis revisited: Current concepts and controversies of an old disease. J Int Assoc Provid AIDS Care. 2019. 18: 2325958219867315
29. Zhang J, Liu X, Fu K, Xu C, Gong R, Liu L. Diagnostic value and safety of stereotactic biopsy in acquired immune deficiency syndrome patients with intracranial lesions: Systematic review and meta-analysis. World Neurosurg. 2017. 98: 790-9.e713