- Department of Neurosurgery, Cork University Hospital, Cork, Ireland.
DOI:10.25259/SNI_129_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lena Mary Houlihan, Charlie Marks. Cerebrospinal fluid hydrodynamics in arachnoid cyst patients with persistent idiopathic intracranial hypertension: A case series and review. 08-Aug-2020;11:237
How to cite this URL: Lena Mary Houlihan, Charlie Marks. Cerebrospinal fluid hydrodynamics in arachnoid cyst patients with persistent idiopathic intracranial hypertension: A case series and review. 08-Aug-2020;11:237. Available from: https://surgicalneurologyint.com/surgicalint-articles/10189/
Abstract
Background: A clear connection has been established between arachnoid cysts (ACs) and the evolution of idiopathic intracranial hypertension (IIH), a connection, which is presently not well understood. Cerebrospinal fluid (CSF) is an integral element of this condition. Little is known about either the influence of AC on CSF hydrodynamics or the specific nature of CSF, which contributes to the complex pathology of IIH.
Case Description: This study aimed to chronicle in detail four patients with previously treated intracranial ACs, who developed persistent IIH. This series and review aims to identify and qualitatively analyze the multiple constituents, which could possibly elucidate the intrinsic relationship between arachnoid cyst-induced IIH and CSF hydrodynamics. A retrospective analysis of the medical records of four patients admitted to the institution’s neurosurgery department during the period of 1994–2013 was completed. This study investigated discernible aspects linking CSF pathophysiology with the development of IIH in AC patients. Four male patients, ranging from 3 to 44 years of age at presentation, had a left-sided arachnoid cyst treated surgically. All four patients subsequently developed IIH. Three patients remain persistently symptomatic.
Conclusion: IIH associated with AC is a hydrodynamic disorder. The full discovery of its fluctuant pathophysiology is the only way to identify an effective standard for the management and treatment of this condition.
Keywords: Arachnoid cyst, Cerebrospinal fluid, Hydrodynamic, Idiopathic intracranial hypertension, Intracranial pressure
BACKGROUND
Idiopathic intracranial hypertension (IIH) is defined as both the clinical and neurological manifestation of increased intracranial pressure (ICP) in the absence of any secondary cause.[
The annual incidence of IIH is 1–2/100,000.[
Arachnoid cysts (ACs) are congenital collections of benign fluid contained within the arachnoid membrane, lined by arachnoid cells, and situated in the subarachnoid space of the cisterns and major cerebral fissures.[
CSF physiology is based on the concept of maintaining hydrodynamic order. Hydrodynamic order, in turn, entails preserving a balance between CSF production, circulation, and reabsorption as well as compensating for deviations by appropriately readjusting the unaffected parameters so as to restore equilibrium between formation and absorption.[
Although IIH by definition is idiopathic, research has postulated a link between IIH and multiple disorders. In the literature, there are various cases documenting this ambiguous connection.[
CSF disturbance has been identified as an important constituent in the development of IIH. It has also been proposed as the sole cause of the disorder and multiple hypotheses have postulated the etymology.[
This retrospective case review series describes four patients admitted during the period of 1994–2013 suffering with IIH in the setting of AC. This study investigated and analyzed the clinical aspects linking CSF pathophysiology with the development of IIH in patients who had AC treated surgically.
CASE REPORT
Case report 1
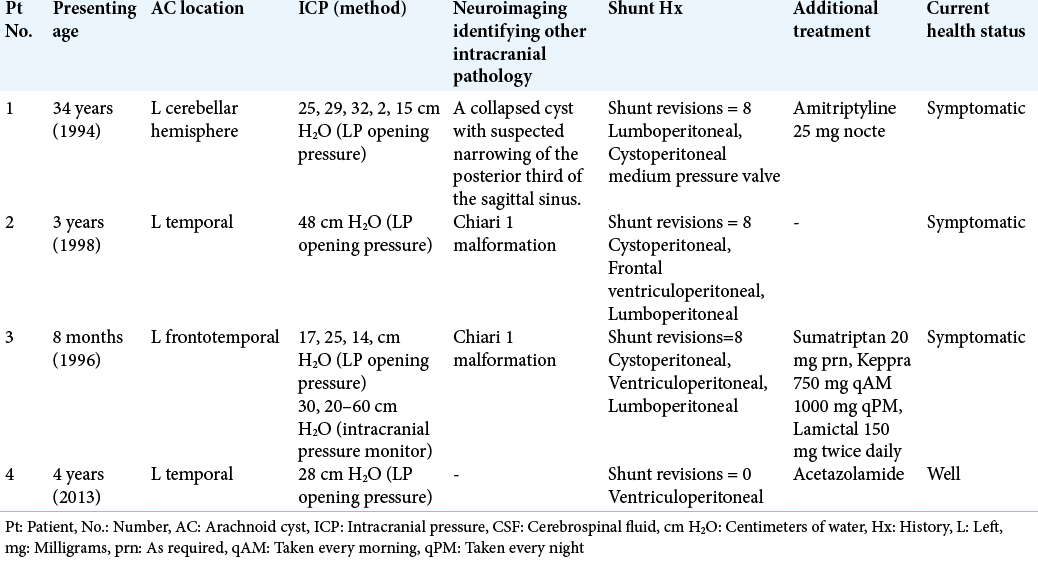
A 34-year-old man presented with headache of gradual onset, which continued intermittently over the subsequent fortnight. The pain was most severe over the left frontal region radiating down the left side of his face, associated with dizziness. Computed tomography (CT) brain identified a superficial AC over the surface of the left cerebellar hemisphere with no mass effect or evidence of hydrocephalus. The patient underwent a burr hole craniotomy, cyst drainage, and excision of a portion of the cyst wall. CSF pressure in the cyst upon measurement was 25 cm H2O.
The patient’s symptoms had not resolved 3 months postoperatively and a follow-up CT revealed reoccurrence of the AC. A posterior fossa craniotomy cyst drainage and more extensive excision of the cyst wall were undertaken. CSF pressure postoperatively was persistently 25 cm H2O.
The patient did not recover well from the second surgery and presented 6 months later with low-grade meningitis, worsening headache, fever, nausea, and vomiting. Lumbar puncture (LP) was performed and revealed an elevated white blood cell count with no organisms, for which he was treated with a course of antibiotics. The patient’s severe headaches persisted.
LP was performed 3 months later, which found a CSF pressure of 29 cm H2O. MRI and magnetic resonance venography (MRV) demonstrated a collapsed cyst with no evidence of venous sinus thrombosis (VST) or any other changes suggestive of increased ICP. Papilledema was not present. A diagnosis of IIH was established and a lumboperitoneal shunt was inserted. The patient’s symptoms improved, and he was subsequently prescribed amitriptyline 25 mg at night, to alleviate any periodic headache.
The patient presented 5 years later with severe occipital headache, dizziness, and vertigo. LP showed a CSF pressure of 32 cm H2O. MRI and MRV demonstrated no reoccurrence of the superficial AC with suspected narrowing of the posterior third of the sagittal sinus. The lumboperitoneal shunt was replaced with a medium pressure valve cystoperitoneal shunt.
Three years later, the patient presented with dizziness following two episodes of severe vertigo accompanied by nausea and vomiting. A further MRI revealed no abnormality apart from the previously identified collapsed AC. On shunt flushing, a CSF pressure of 2 cm H2O was noted. Low-pressure symptomatology was identified. A high-pressure valve was inserted. This further aggravated the patient’s symptoms. CSF pressure was 15 cm H2O on measurement and further valve pressure downgrading was achieved by insertion of a variable pressure shunt set at 7 cm H2O replacing the high-pressure shunt. This patient remains persistently symptomatic.
Case report 2
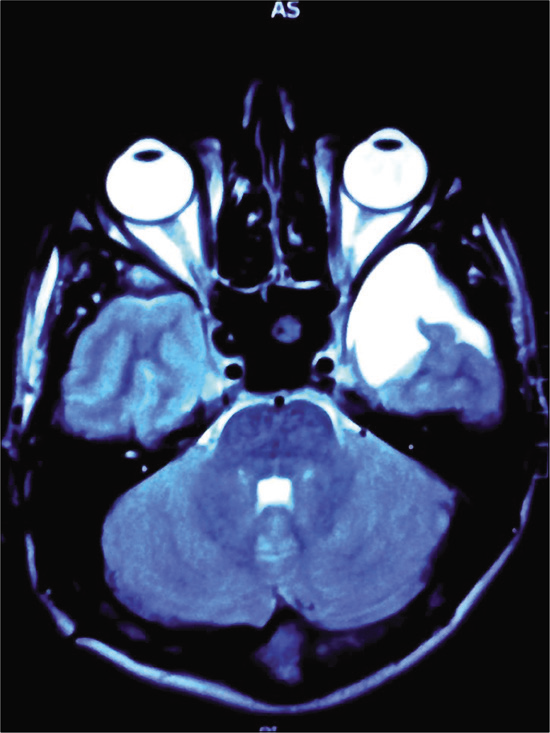
A left-sided temporal AC [
This cyst remained benign until the age of 3, on which the patient presented with a 3-week history of progressively worsening headache, vomiting, lethargy, and irritability. No papilledema was noted. A cystoperitoneal shunt was inserted. A postoperative CT revealed a collapsed cyst and the patient experienced symptomatic relief.
At 7 years of age, the patient presented with episodic severe headache and vomiting and no other signs or symptoms. CT scan revealed no evidence of cystic fluid collection and the ventricles were normal in size, shape, and position. The patient was treated symptomatically, and his complaints resolved.
The patient presented at 11 years of age to the emergency department with diplopia, photophobia, headache, and vomiting. Bilateral abducens nerve palsy was identified with bilateral papilledema noted on fundoscopy. CT scan revealed slit ventricles and no residual temporal AC. A cystoperitoneal shunt was in situ and no malfunction was identified. MRI scan showed a Chiari malformation. There were no associated syrinx or tussive headaches in the occipital region on coughing or straining.
Intracranial hypertension without dilated ventricles was diagnosed. The cystoperitoneal shunt was revised, but when the patient showed no improvement, a right frontal ventriculoperitoneal shunt was inserted which appeared to relieve the patient’s symptoms. The patient presented with two further symptomatic episodes due to shunt malfunction. A new lumboperitoneal shunt was inserted, and the symptoms resolved, although multiple shunt revisions were necessary.
A year later, the patient presented with a short history of recurrent headache, for which the lumboperitoneal shunt was revised. CT venogram showed patent venous sinuses with normal deep venous system and no evident stenosis. The diagnosis of IIH was reaffirmed.
At 17 years of age, the patient underwent foramen magnum decompression for a Chiari malformation and presented 3 weeks later with severe headaches. LP revealed a CSF pressure of 48 cm H2O and slit-ventricle syndrome. A right- sided ventriculoperitoneal catheter was inserted, and his symptoms improved. To date, this patient has undergone eight shunt revision procedures.
Case report 3
An 8-month-old male presented with a short history of absence seizures. A large left frontotemporal AC was identified on CT scan with no mass effect or evidence of hydrocephalus. A cystoperitoneal shunt was inserted. This resulted in complete resolution of the cyst.
Over the following 10 years, the patient presented with migraine-like attacks with no contributing evidence on CT scan and no residual cyst formation. He was treated with intranasal sumatriptan 20 mg prn to be administered on the onset of headaches.
At 13 years of age, the patient was referred after a persistent headache of 1 week’s duration and three episodes of vomiting. No papilledema was noted on fundoscopy. LP revealed CSF pressure of 17 cm H2O. The patient continued to suffer with episodic severe headache and seizures. The patient went on to develop blurred vision with normal visual acuity and bilateral papilledema. CT scan revealed slit ventricles and no evidence of cyst reoccurrence. ICP monitor recorded consistently high pressures ranging from 20 cm to 60 cm H2O. The patient was diagnosed with IIH and a ventriculoperitoneal shunt was inserted.
The patient experienced reoccurrence of headaches 8 months later. LP showed a CSF pressure of 25 cm H2O. A cephalic block was identified, and a new ventriculoperitoneal catheter was inserted along the track of its predecessor. The patient’s slit-ventricle syndrome persisted.
The patient became symptomatic 5 months later. LP showed a pressure of 25 cm H2O. A lumboperitoneal shunt was inserted. The patient subsequently developed low-pressure symptoms – headaches worse during the day instantly relieved by lying down. A high-pressure valve replaced the low-medium pressure Hakim valve. The patient was well for a week, until sudden onset of worsening severe headaches. LP showed CSF pressure of 14 cm H2O, a pressure apparently too high for the patient to tolerate. A medium-high pressure Hakim valve replaced the high-pressure valve.
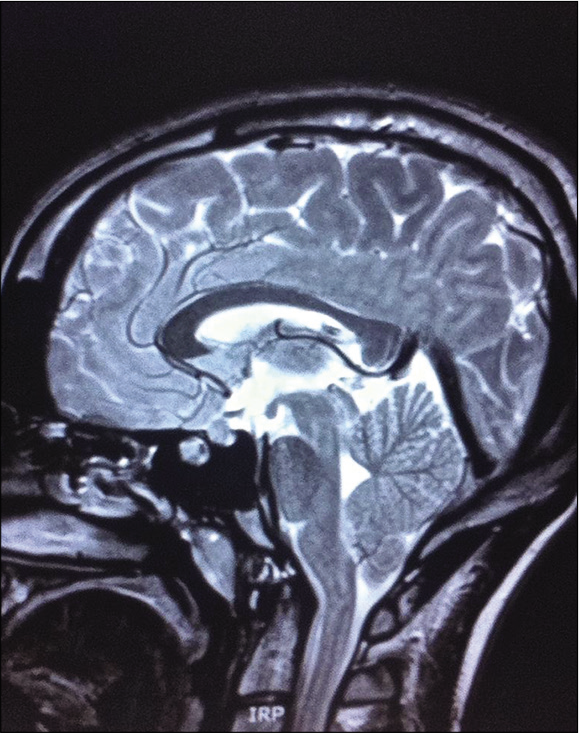
The patient continued to experience persistent, if not milder episodic headaches. MRI identified a Chiari malformation with cerebellar tonsillar protrusion approximately 1 cm below the level of the foramen magnum [
At 16 years of age, the patient had three complex partial seizures. An electroencephalogram showed excess intermittent rhythmical slow-wave activity over the left hemisphere. This implied the potential for partial and generalized seizures, findings consistent with an underlying structural abnormality in the left temporal region, the area of the collapsed AC. The patient was treated with Keppra, 750 mg qAM and 1000 mg qPM and Lamictal, 50 mg twice daily.
The patient remained reasonably well for 2 years. He once more experienced similar episodes of headache, vomiting, and tonic seizures. ICP monitor identified markedly raised pressure of a minimum of 30 cm H2O. The lumboperitoneal shunt was removed and a new cystoperitoneal shunt was inserted.
Case report 4
A 4-year-old male presented with intermittent severe headache and irritability. On physical examination, a substantial left-sided temporal bulge was found. CT scan identified a large left temporal AC with no mass effect or evidence of hydrocephalus.
The patient’s headaches continued to worsen, and a craniotomy was undertaken with excision of the cyst wall. There was moderate symptomatic improvement for 1 month. A repeat CT scan showed that the cyst had decreased in size by 50%. A trial of acetazolamide was prescribed, which produced little symptomatic improvement. LP revealed a CSF pressure of 28 cm H2O. A ventriculoperitoneal shunt was inserted. The patient is currently well.
DISCUSSION
There are conflicting views as to the composition of AC fluid. It was thought that arachnoid cells lining the cyst secrete CSF, but recent chemical analysis has revealed that AC fluid is not identical to CSF.[
This compositional difference between AC fluid and CSF is relevant as it presents an alternative mechanism of cystic fluid accumulation. This evidence supports the concept of active transport as a mechanism underlying AC filling.[
It must be questioned whether this mechanism modifies the composition and substrate concentration of CSF. In turn, such changes in CSF could modify the rate or amount of fluid absorbed by the AV, thus causing an elevation in ICP, a possible causal agent or compounding factor leading to, or augmenting IIH. Although there is no demonstration of this concept in the above cases, it is an idea worth discussing in this review.
Factors that influence the production of CSF can be classified as exogenous or endogenous. Neural influences affecting fluid production include sympathetic, cholinergic, and peptidergic fibers.[
Approximately 15–20 ml of CSF is produced every hour. An increase in CSF volume can be due to increased production but can also be associated with hydrostatic, hydrodynamic, and obstructive mechanisms as a possible reason for elevated CSF pressure.[
Assessing volume is complex and difficult.[
Although an established entity in human adults, AV are not found in the human infant.[
If the AV exist in an environment, which include cyst-like masses and adjust accordingly during development, what effect does obliteration of the cyst have on the villi? The presence of an AC can be considered a structural abnormality and a potential obstruction, modifying fluid circulation, and absorption. As discussed in relation to AV and absorption, the embryonic origin of such cysts undoubtedly alters orthodox CSF anatomy and possibly its physiology.
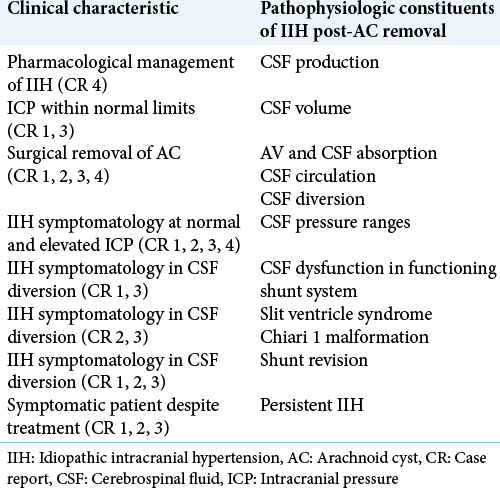
Patients 1, 2, 3, and 4 all underwent surgical intervention to treat AC intending to alleviate symptoms [
VST is also recognized as a possible cause, consequence, or compounding factor in the development of IIH. Patient 1 exhibited narrowing of the posterior third of the sagittal sinus on MRV, which was detected 6 years after initial symptomatic presentation. Including and identifying this agent are noteworthy, but it does not contribute to the present discussion, as there is no known association between AC and venous sinus dysfunction.
CSF pressure values and assessment formulate our present concept of CSF equilibrium, the balance between the production and absorption of CSF. The most recent revision of the Dandy criteria diagnoses IIH in the presence of signs and symptoms of elevated ICP with a CSF opening pressure of >25 cm H2O, normal CSF constituents, and no alternative explanation of increased ICP.[
Our guiding CSF pressure range of normal can be misleading on the backdrop of IIH, which can manifest symptomatic disequilibrium at various fluid pressures as is evident in Patients 1, 2, 3, and 4. As depicted in the reported cases, IIH may present in the presence of what is classified as both increased and normal CSF pressure. Volume usually correlates directly with pressure, but this relationship does not necessarily correlate with the manifestation of IIH.
Rate of CSF production (15–20 ml/h) has been acknowledged as acting independently to CSF pressure levels except in cases of severely elevated cerebral pressures.[
Shunt success
Conceptually, shunting is the most direct and logical method of rapidly decreasing elevated ICP. CSF shunting can be achieved through various different diversion pathways. Despite the multiple potential complications associated with shunting, it is by far the most commonly used treatment in IIH and its incidence is rising.[
CSF diversion as a treatment option in AC patients creates shunt dependency.[
Although shunt complications and malfunction are a very prevalent possibility, repeated CSF divertive action was undertaken by the neurosurgery department aimed to relieve elevated ICP. As seen in Patients 1, 2, and 3, symptomatic episodes did occur in the presence of no shunt dysfunction, yet alleviation of ICP was necessary. At present, it is rational to suggest that the most therapeutic method of improving a symptomatic patient is through CSF diversion techniques.
Slit-ventricle syndrome
Slit-ventricle syndrome is the decrease in size or collapse of the ventricles on radiological imaging.[
Shunt revision
Shunt systems are unreliable, difficult to evaluate from a functional level,[
Out of the four patients listed, above three required surplus CSF diversion. This idea is further perpetuated by the negative consequences of indirect shunting, including ventricle collapse and its sequelae slit-ventricle syndrome, low-pressure syndromes, and structural displacement, most specifically, the acquired Chiari malformation, as seen in Patients 2 and 3.
Indirect shunting aims to ameliorate increased ICP and restore equilibrium. In actuality, the procedure may inadvertently exacerbate a previously established hydrodynamic disorder.
Patients 1, 2, and 3 all remain symptomatic despite multiple and varied therapeutic efforts. Although the respective treatments appear successful and provide symptom relief, the respite period is finite, and the patients once more become symptomatic. The persistence of the condition may be attributed, to on-going issues with indirect shunting, limited treated options, or simply a failure to ascertain a fully efficacious treatment.
This continuous transformation strengthens the link between persistent progressive IIH and the hydrodynamic status of CSF. The advancing nature of the disease also authenticates the inability to decipher a solid evidence-based strategy toward the management of IIH, as our present view identifies IIH as a static condition. The balancing act and continuous remodeling demonstrated with therapeutic CSF diversion as seen in Patients 1, 2, and 3, reinforced the idea of IIH as a dynamic evolving disorder.
This study is a retrospective case series. The study is limited as it allows for documentation and commentary on the patients and their condition. It is only useful for descriptive, exploratory analysis of the patients who have this disorder. It is also unknown whether these results are applicable to a wider population. Furthermore, replication is difficult. Researcher bias is also present as the researchers can only assess the data subjectively.
The patient ages ranged from 3 to 44 years. This is the largest series to date of patients suffering with IIH in the presence of AC, although the sample size is small. This is due to the extremely rare nature of the condition, as well as the collected sample being limited to patients attending the neurosurgery department from 1994 to 2013. This leads to issues with interpretation of the qualitative analysis. Regardless, this case series provides rich, qualitative data on a condition, and obscure association where there is, as of yet, very little knowledge and understanding. This study is not designed to result in strong conclusions; rather, these data should be applied to the format of larger confirmatory studies and testable hypotheses.
This study attempts to link anatomical, physiological, and pathophysiological principles with the patients’ clinical characteristics [
CONCLUSION
If we can infer anything from the above evidence, it is the irrefutable fact that IIH, in the setting of AC, is a hydrodynamic condition of fluctuant nature. Identification of this disequilibrium’s pathophysiology in AC patients will result in an efficacious treatment and subsequently a resolution of IIH.
This review increases our depth of knowledge into this obscure condition and links clinical characteristics with various anatomical and pathophysiological elements to enhance our understanding. It also highlights how much there is yet to be discovered.
A clear guide to the management of this condition can only be definitively established when the pathophysiologic etiology is fully understood. It is for this reason that further physiological studies of AC and CSF hydrodynamics are necessary in this field.
IIH is a diagnosis of exclusion. It is so, because much has still to be clarified about the condition. Conceivably, the cause of IIH may never be discovered. This condition encompasses all disorders of elevated ICP and until the origin and pathological mechanisms are gradually illuminated, these illnesses will remain classified as idiopathic.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albuquerque FC, Giannotta SL. Arachnoid cyst rupture producing subdural hygroma and intracranial hypertension: Case reports. Neurosurgery. 1997. 41: 951-6
2. Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in children: Clinical article. J Neurosurg Pediatr. 2010. 5: 578-85
3. Aschoff A, Kremer P, Benesch C, Fruh K, Klank A, Kunze S. Overdrainage and shunt technology. Childs Nerv Syst. 1995. 11: 193-202
4. Berle M, Wester KG, Ulvik RJ, Kroksveen AC, Haaland O, Amiry-Moghaddam M. Arachnoid cysts do not contain cerebrospinal fluid: A comparative chemical analysis of arachnoid cyst fluid and cerebrospinal fluid in adults. Cerebrospinal Fluid Res. 2010. 7: 8
5. Chari C, Rao NS. Benign intracranial hypertension-its unusual manifestations. Headache. 1991. 31: 599-600
6. Corbett JJ, Savino PJ, Thompson HS, Kansu T, Schatz NJ, Orr LS. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982. 39: 461-74
7. Curry WT, Butler WE, Barker FG. Rapidly rising incidence of cerebrospinal fluid shunting procedures for idiopathic intracranial hypertension in the United States 1988-2002. Neurosurgery. 2005. 57: 97-108
8. Cushing H. Studies on the cerebro-spinal fluid: I. Introduction. J Med Res. 1914. 31: 1-19
9. Cutler R, Page L, Galicich J, Watters G. Formation and absorption of cerebrospinal fluid in man. Brain. 1968. 91: 707-20
10. Dandy WE. Intracranial pressure without brain tumor: Diagnosis and treatment. Ann Surg. 1937. 106: 492-513
11. Davidoff LM. Pseudotumor cerebri; benign intracranial hypertension. Neurology. 1956. 6: 605-15
12. Davson H, Segal MB.editors. Physiology of the CSF and Blood-Brain Barriers. Boca Raton: CRC Press; 1996. p.
13. Degnan AJ, Levy LM. Pseudotumor cerebri: Brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011. 32: 1986-93
14. Donahue SP. Recurrence of idiopathic intracranial hypertension after weight loss: The carrot craver. Am J Ophthalmol. 2000. 130: 850-1
15. Durcan PJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri: Population studies in Iowa and Louisiana. Arch Neurol. 1988. 45: 875-7
16. Eggenberger ER, Miller NR, Vitale S. Lumboperitoneal shunt for the treatment of pseudotumor cerebri. Neurology. 1996. 46: 1524-30
17. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002. 59: 1492-5
18. Friedman DI. Medication-induced intracranial hypertension in dermatology. Am J Clin Dermatol. 2005. 6: 29-37
19. Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri) a case-control study. Neurology. 1991. 41: 239-44
20. Greenfield DS, Wanichwecharungruang B, Liebmann JM, Ritch R. Pseudotumor cerebri appearing with unilateral papilledema after trabeculectomy. Arch Ophthalmol. 1997. 115: 423-6
21. Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension: A preliminary case-control study. Arch Neurol. 1990. 47: 315-20
22. Jain N, Rosner F. Idiopathic intracranial hypertension: Report of seven cases. Am J Med. 1992. 93: 391-5
23. Johnston I, Teo C. Disorders of CSF hydrodynamics. Childs Nerv Syst. 2000. 16: 776-99
24. Kaliaperumal C, O’Connor B, Marks C. Development of intracranial hypertension after surgical management of intracranial arachnoid cyst: Report of three cases and review of the literature. World Neurosurg. 2013. 80: 222.e1-4
25. Kesler A, Gadoth N. Epidemiology of idiopathic intracranial hypertension in Israel. J Neuroophthalmol. 2001. 21: 12-4
26. Kesler A, Goldhammer Y, Gadoth N. Do men with pseudomotor cerebri share the same characteristics as women? A retrospective review of 141 Cases. J Neuroophthalmol. 2001. 21: 15-7
27. Kim SK, Cho BK, Chung YN, Kim HS, Wang KC. Shunt dependency in shunted arachnoid cyst: A reason to avoid shunting. Pediatr Neurosurg. 2002. 37: 178-85
28. Le GW. On the Pacchionian bodies. J Anat. 1920. 55: 40-8
29. Lorenzo AV, Page LK, Watters GV. Relationship between cerebrospinal fluid formation, absorption and pressure in human hydrocephalus. Brain. 1970. 93: 679-92
30. Maixner VJ, Besser M, Johnston IH. Pseudotumor syndrome in treated arachnoid cysts. Childs Nerv Syst. 1992. 8: 207-10
31. Nilsson C, Lindvall-Axelsson M, Owman C. Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res Rev. 1992. 17: 109-38
32. Quintana LM. An unresolved relationship-treated arachnoid cysts and idiopathic intracranial hypertension. World Neurosurg. 2013. 80: 80-2
33. Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O’Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri) descriptive epidemiology in Rochester Minn 1976 to 1990. Arch Neurol. 1993. 50: 78-80
34. Rengachary S, Kennedy J. Intracranial arachnoid and ependymal cysts. Neurosurgery. New York: McGraw-Hill; 1985. 3: 2160-72
35. Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981. 40: 61-83
36. Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966. 25: 430-6
37. Sinclair AJ, Kuruvath S, Sen D, Nightingale PG, Burdon MA, Flint G. Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia. 2011. 31: 1627-33
38. Skau M, Brennum J, Gjerris F, Jensen R. What is new about idiopathic intracranial hypertension? An updated review of mechanism and treatment. Cephalalgia. 2006. 26: 384-99
39. Tripathi RC. The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res. 1977. 25: 65-116
40. Trost H, Heissler H, Claussen G, Gaab M. Testing the hydrocephalus shunt valve: Long-term bench test results of various new and explanted valves. The need for a model for testing valves under physiological conditions. Eur J Pediatr Surg. 1991. 1: 38-40
41. Vischi A, Guerriero S, Giancipoli G, Lorusso V, Sborgia G. Delayed onset of pseudotumor cerebri syndrome 7 years after starting human recombinant growth hormone treatment. Eur J Ophthalmol. 2006. 16: 178-80
42. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 Patients. Brain. 1991. 114: 155-80
43. Wall M, White W. Asymmetric papilledema in idiopathic intracranial hypertension: Prospective interocular comparison of sensory visual function. Invest Ophthalmol Vis Sci. 1998. 39: 134-42
44. Weed LH. An anatomical consideration of the cerebrospinal fluid. Anat Rec. 1917. 12: 461-96
45. Weed LH. Forces concerned in the absorption of the cerebrospinal fluid. Am J Physiol Leg Content. 1935. 114: 40-5
46. Whiteley W, Al-Shahi R, Warlow C, Zeidler M, Lueck C. CSF opening pressure: Reference interval and the effect of body mass index. Neurology. 2006. 67: 1690-1






James Ausman
Posted September 6, 2020, 7:17 am
Very nice review of a complicated problem. Must be agony for the patient. It is for the physician, also. Multifactorial issues in your cases: 1) technical complications with shunt failures, 2) CSF & Fluid dynamic issues & Meningitis. LP shunts have high failure rate. 3) Brain issues with Slit ventricles & Chiari. Have you analyzed the CSF protein levels? Might be a unifying cause. What about Isotope CSF studies to check flow dynamics?
Very troubling.
Thanks for submitting this.