- Department of Neurosurgery, “Riuniti” Hospital, Foggia, Italy.
- Department of Neurosurgery, University of Foggia, Foggia, Puglia, Italy.
Correspondence Address:
Francesco Carbone, Department of Neurosurgery, University of Foggia, Foggia, Puglia, Italy.
DOI:10.25259/SNI_950_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Matteo Sacco1, Savino Iodice1, Nicola Pio Fochi2, Francesco Carbone2. Cerebrospinal fluid leak as a driving factor in chronic subdural hematoma formation: A histological study. 23-Nov-2021;12:578
How to cite this URL: Antonio Colamaria1, Matteo Sacco1, Savino Iodice1, Nicola Pio Fochi2, Francesco Carbone2. Cerebrospinal fluid leak as a driving factor in chronic subdural hematoma formation: A histological study. 23-Nov-2021;12:578. Available from: https://surgicalneurologyint.com/surgicalint-articles/11237/
Abstract
Background: Chronic subdural hematoma (CSDH) represents the most common neurosurgical disease. Given the demographic shift toward an aging population, the overall incidence of this condition is increasing. Nevertheless, clarity in the pathophysiological process is yet to be made. Several etiological mechanisms have been proposed to initiate and consequently promote fluid collection in the subdural space. Traumatic injury of the bridging veins has long been considered the primum movens of the pathology but increasing evidence shows that trauma is not the only factor involved. Along with recent advances we sought to understand the role of the cerebrospinal fluid (CSF) in the buildup of the intense inflammatory reaction that characterizes CSDH.
Methods: In the present study, we examined histological features of reactive membranes secondary to extracranial CSF leakage with CSDH-related membranes. Similarity and differences between the specimens were examined by means of light microscopy.
Results: Histological similarities were consistently found between CSDH membranes and reactive membranes secondary to CSF leakage in the extracranial space. Activated histiocytes were highlighted in all specimens along with an intense inflammatory reaction.
Conclusion: CSDH is most likely the result of a complex interaction among different pathophysiological events resulting from both traumatic and inflammatory etiologies. In the present work, we highlight how CSF leakage could be an early factor that leads to a cascade of events that culminates in CSDH formation.
Keywords: Cerebrospinal fluid, Chronic subdural hematoma, Dura mater, Inflammation, Subdural hygroma
INTRODUCTION
Chronic subdural hematoma (CSDH) is a pathological collection of fluid in the subdural space. It is recognized as one of the most frequently encountered neurosurgical diseases,[
Traumatic injury was first suggested as the main cause of CSDH formation, linking the tearing of the bridging veins to the accumulation of venous blood layered between the arachnoid and dura mater.[
The scope of the present study was to examine histological specimens derived from reactive membranes secondary to cerebrospinal fluid (CSF) leakage in the abdominal wall and CSDH-related membranes found in patients treated with burr hole surgery.
MATERIALS AND METHODS
Patient selection
During the months of March and July 2021, two cases of ventriculoperitoneal shunt malfunction associated with CSF leakage in the abdominal wall were admitted to our institution. Both these patients were previously treated for idiopathic normal pressure hydrocephalus. At 1-month follow-up fluid in the subcutaneous adipose tissue was noticed, indicating that shunt had pull out of the subperitoneal space. During surgical revision, reactive membranes were obtained.
In the same period, two cases of post-craniotomy subgaleal CSF collection were identified. Revision surgery for dural fistula allowed the collection of subcutaneous reactive membranes caused by CSF leakage. These patients were not treated for CSDH and came to our attention due to primary CNS tumors managed with surgery.
Between August and November 2020, five patients were admitted with the diagnosis of CSDH. The first three patients presented with clinical and radiological evidence of CSDH and were thus admitted and treated with single burr hole surgery. The fourth and fifth patients exhibited evidence of subdural hygroma (SDHy) on computed tomography (CT) scan and were managed with a “wait and watch” strategy as most of these patients usually improve over time. After initial improvement, all patients came back in 2021 with worsened symptoms, and an evacuative craniotomy was performed. Reactive membranes were obtained during surgery.
The patients enlisted in this study did not show any relevant neurosurgical condition in addition to those for which they were treated, and their characteristics are summarized in [
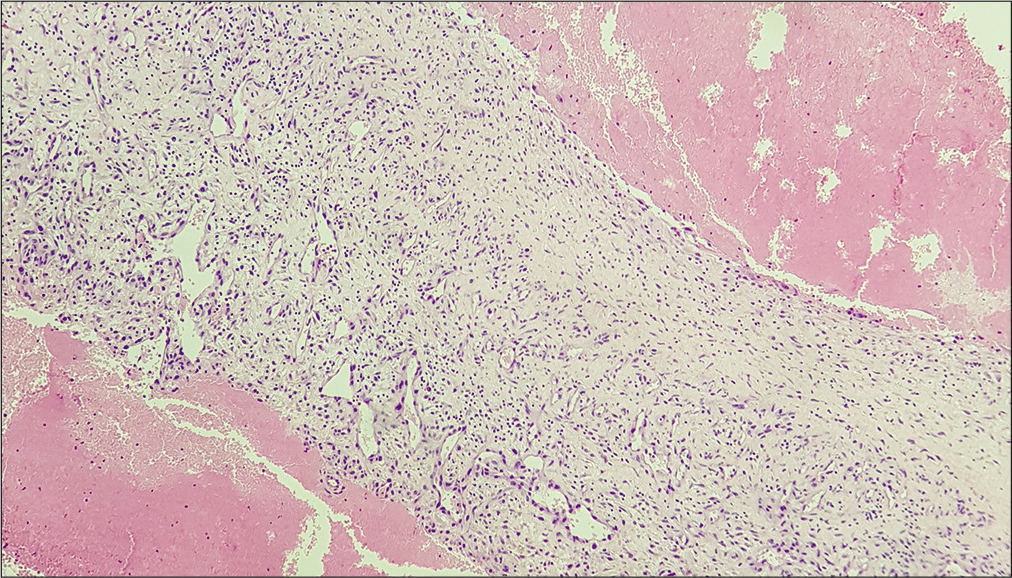
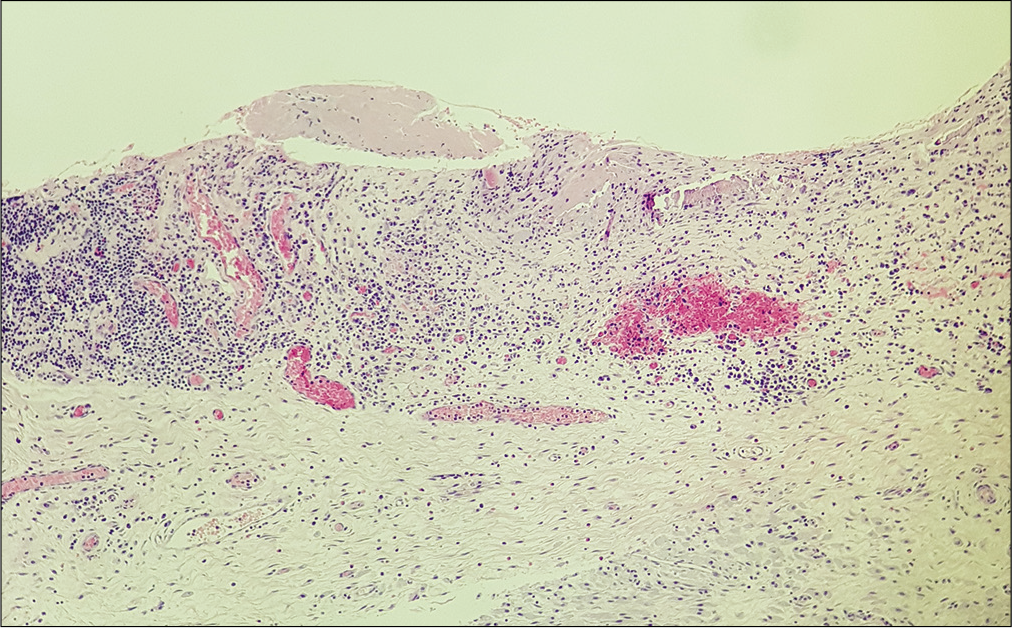
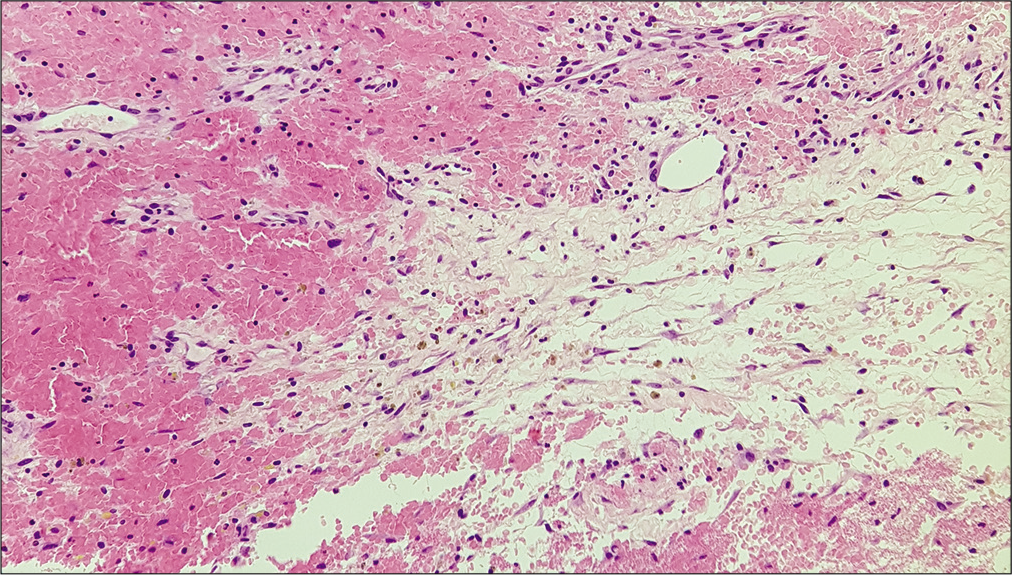
Histological analysis was carried out using light microscopy. Independent and comparative evaluation of the nine membranes allowed the identification of similarities. Different areas of each membrane were evaluated to exclude possible sampling bias.
RESULTS
Histological examination of the membrane tissue revealed a substantial non-specific inflammatory reaction in both reactive and CSDH-related specimens. In each type of membrane, collagen fibers and different layers of fibroblasts were noticed along with neutrophils and dural histiocytes [
DISCUSSION
From trauma to drama
CSDH has been recently reconsidered as part of an intense and sustained inflammatory reaction rather than a post-traumatic injury alone.[
Mediators involved
Bleeding hosts a central role in the pathogenesis of CSDH and recently several angiogenic mediators have been identified to be involved in the formation of the reactive membranes. The concentration of VEGF and its receptor is reported to be more than 28 times higher in the subdural fluid collection than that found in serum.[
Role of CSF leakage
Recent evidence shows how CSF leakage in the subdural space could be associated with CSDH formation.[
In the present work, we speculate that CSF leakage into extracranial sites could initiate a similar inflammatory reaction to the one associated with CSDH formation, therefore indicating that reactive membranes could be secondary to a non-specific inflammatory reaction associated with the subdural CSF leakage. Findings of similarities between reactive and CSDH-related specimens suggest that CSF could be an underestimated factor in the formation of the membranes. The presence of activated histiocytes could also indicate a common mechanism underlying the two processes. If this is confirmed by future research, CSF leakage in the subdural space could not only be associated with hygroma but also, under certain conditions, cause progression to CSDH.
Future treatment perspectives
Knowledge of the pathophysiological dynamics of CSDH is crucial for optimizing surgical and pharmacological treatment options. Operative treatment has long been considered the main solution for the patients presenting neurological deterioration and radiological evidence of CSDH.[
CONCLUSION
CSDH is likely the result of a complex interaction among various biomolecular pathways induced by both traumatic injury and inflammatory dysregulation. In rare cases, it can result from an SDHy progressed the most of the time with no clinical evidence. The role of the immune system and particularly histiocytes is still debated. In the present work, we found evidence of non-specific inflammatory reaction associated with extracranial CSF leakage and speculate that the histological similarities seen with the CSDH-related membranes could indicate an underlined common pathophysiological mechanism.
Further research is necessary to better understand phlogistic reactions caused by CSF leakage in the subdural space and how these could influence pharmacological therapy.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. D’Abbondanza JA, Loch Macdonald R. Experimental models of chronic subdural hematoma. Neurol Res. 2014. 36: 176-88
2. Edlmann E, Whitfield PC, Kolias A, Hutchinson PJ. Pathogenesis of chronic subdural hematoma: A cohort evidencing de novo and transformational origins. J Neurotrauma. 2021. 38: 2580-9
3. Funai M, Osuka K, Usuda N, Atsuzawa K, Inukai T, Yasuda M. Activation of PI3 kinase/Akt signaling in chronic subdural hematoma outer membranes. J Neurotrauma. 2011. 28: 1127-31
4. Gelabert-González M, Iglesias-Pais M, García-Allut A, Martínez-Rumbo R. Chronic subdural haematoma: Surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005. 107: 223-9
5. Hara M, Tamaki M, Aoyagi M, Ohno K. Possible role of cyclooxygenase-2 in developing chronic subdural hematoma. J Med Dent Sci. 2009. 56: 101-6
6. Heula AL, Sajanti J, Majamaa K. Procollagen propeptides in chronic subdural hematoma reveal sustained Dural collagen synthesis after head injury. J Neurol. 2009. 256: 66-71
7. Hohenstein A, Erber R, Schilling L, Weigel R. Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and-2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005. 22: 518-28
8. Inglis K. Subdural haemorrhage, cysts and false membranes; illustrating the influence of intrinsic factors in disease when development of the body is normal. Brain. 1946. 69: 157-94
9. Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976. 45: 26-31
10. Kalamatianos T, Stavrinou LC, Koutsarnakis C, Psachoulia C, Sakas DE, Stranjalis G. PlGF and sVEGFR-1 in chronic subdural hematoma: Implications for hematoma development. J Neurosurg. 2013. 118: 353-7
11. Kristof RA, Grimm JM, Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space: Possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma. J Neurosurg. 2008. 108: 275-80
12. Leroy HA, Aboukaïs R, Reyns N, Bourgeois P, Labreuche J, Duhamel A, Lejeune JP. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci. 2015. 22: 1895-900
13. Markwalder TM. Chronic subdural hematomas: A review. J Neurosurg. 1981. 54: 637-45
14. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci. 2018. 50: 7-15
15. Miranda LB, Braxton E, Hobbs J, Quigley MR. Chronic subdural hematoma in the elderly: Not a benign disease. J Neurosurg. 2011. 114: 72-6
16. Moskala M, Goscinski I, Kaluza J, Polak J, Krupa M, Adamek D. Morphological aspects of the traumatic chronic subdural hematoma capsule: SEM studies. Microsc Microanal. 2007. 13: 211-9
17. Nanko N, Tanikawa M, Mase M, Fujita M, Tateyama H, Miyati T. Involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor in the mechanism of development of chronic subdural hematoma. Neurol Med Chir (Tokyo). 2009. 49: 379-85
18. Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994. 81: 910-3
19. Ommaya AK, Yarnell P. Subdural haematoma after whiplash injury. Lancet. 1969. 2: 237-9
20. Park CK, Choi KH, Kim MC, Kang JK, Choi CR. Spontaneous evolution of posttraumatic subdural hygroma into chronic subdural haematoma. Acta Neurochir (Wien). 1994. 127: 41-7
21. Petralia CC, Manivannan S, Shastin D, Sharouf F, Elalfy O, Zaben M. Effect of steroid therapy on risk of subsequent surgery for neurologically stable chronic subdural hemorrhage-retrospective cohort study and literature review. World Neurosurg. 2020. 138: e35-41
22. Putnam TJ, Cushing . Chronic subdural hematoma: Its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Arch Surg. 1925. 11: 329-93
23. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019. 132: 1147-57
24. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009. 374: 1067-73
25. Shono T, Inamura T, Morioka T, Matsumoto K, Suzuki SO, Ikezaki K. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci. 2001. 8: 411-5
26. Stroobandt G, Fransen P, Thauvoy C, Menard E. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir (Wien). 1995. 137: 6-14
27. Thotakura AK, Marabathina NR. Nonsurgical treatment of chronic subdural hematoma with steroids. World Neurosurg. 2015. 84: 1968-72
28. Toi H, Fujii Y, Iwama T, Kinouchi H, Nakase H, Nozaki K. Determining if cerebrospinal fluid prevents recurrence of chronic subdural hematoma: A multi-center prospective randomized clinical trial. J Neurotrauma. 2019. 36: 559-64
29. Uno M, Toi H, Hirai S. Chronic subdural hematoma in elderly patients: Is this disease benign?. Neurol Med Chir (Tokyo). 2017. 57: 402-9
30. Wawrose RA, Dombrowski ME, Crasto JA, Shaw JD, Lee JY. Chronic subdural hematoma as a complication of cerebrospinal fluid leak during revision lumbar spine surgery: A case report and review of the literature. HSS J. 2020. 16: 482-4
31. Welling LC, Welling MS, Teixeira MJ, Figueiredo EG. Chronic subdural hematoma: So common and so neglected. World Neurosurg. 2018. 111: 393-4
32. Wetterling T, Demierre B, Rama B, Nekic M. Protein analysis of subdural hygroma fluid. Acta Neurochir (Wien). 1988. 91: 79-82