- Division of Neurosurgery, Department of Surgery, College of Medicine, University of Ibadan, Ibadan, Nigeria
- Department of Anaesthesia, University College Hospital, Ibadan, Nigeria
- Department of Neurosurgery, University College Hospital, Ibadan, Nigeria
Correspondence Address:
James Ayokunle Balogun
Division of Neurosurgery, Department of Surgery, College of Medicine, University of Ibadan, Ibadan, Nigeria
Department of Neurosurgery, University College Hospital, Ibadan, Nigeria
DOI:10.25259/SNI-47-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: James Ayokunle Balogun, Olusola Kayode Idowu, Adefolarin Obanisola Malomo. Challenging the myth of outpatient craniotomy for brain tumor in a Sub-Saharan African setting: A case series of two patients in Ibadan, Nigeria. 24-Apr-2019;10:71
How to cite this URL: James Ayokunle Balogun, Olusola Kayode Idowu, Adefolarin Obanisola Malomo. Challenging the myth of outpatient craniotomy for brain tumor in a Sub-Saharan African setting: A case series of two patients in Ibadan, Nigeria. 24-Apr-2019;10:71. Available from: http://surgicalneurologyint.com/surgicalint-articles/9282/
Abstract
Background:The concept of modern neuro-oncology hinges on strategic innovation and refinement of procedures with the intention to enhance safety, optimize extent of tumor resection, and improve not only survival but also the quality of life as well. One of such refinements includes same-day hospital admission, as well as early discharge following brain tumor surgeries. The latter has been further stretched to same-day discharge in particular settings to reduce the risk of nosocomial infections, cut brain tumor surgery costs, and improve patients’ satisfaction. We highlight the challenges and possible benefits of outpatient craniotomy in a sub-Saharan African setting portrayed by the presence of lean resources and a predominant “out of pocket” health-care financing.
Case Description:Outpatient craniotomy was performed in two selected patients harboring intra-axial tumors: a right temporal low-grade glioma and a left frontal metastasis. The clinical outcome proved successful at short- and long-term in both patients; complications related to surgery and same-day discharge were not reported.
Conclusion:Outpatient craniotomy is practicable and safe in resource-challenged environments and can further make brain tumor surgery cost effective and acceptable in carefully selected patients. Further prospective studies in similar settings but involving larger groups of patients are warranted.
Keywords: Awake surgery, brain tumor, intensive care unit, outpatient craniotomy, sub-Saharan Africa
INTRODUCTION
Surgical neuro-oncology has remarkably evolved through a series of technological innovations and refinement of procedures aimed at maximizing tumor resection (or ablation in the face of radiosurgery and thermal-targeted procedures) and improving patient’s safety while preserving key neurologic functions. The latter breakthroughs have allowed the betterment of preoperative imaging techniques to include physiologic and functional imaging modalities. In the realm of microsurgery, crucial developments have been achieved, with the introduction of adjuncts such as neuronavigation, intraoperative magnetic resonance imaging (MRI), direct cortical and subcortical stimulation mapping, navigable intraoperative ultrasound, fluorescence-guided resection, cavitron ultrasonic aspirator, laser, and neuroendoscopy.[
MATERIALS AND METHODS
The patient selection took place at the neurosurgical outpatient service of a 800-bed hospital, located in an urban setting with a population of about 3 million (University College Hospital, Ibadan, Southwestern Nigeria). The service serves as a referral center to a wide catchment area; most of the patients pay for their care from “out of pocket.” Case selection was structured with institutional approval and according to the criteria suggested by Carrabba et al.[
CASE SUMMARIES
Case background
The first patient was a 36-year-old female, with a prior history of surgery and chemotherapy for a left breast carcinoma about 13 months earlier. She presented with a 6-month history of headaches with intermittent vomiting, progressive bilateral visual loss, and right-sided weakness. Her visual acuity was decreased to perceive only hand movement on the right side and no light perception on the left side with evidence of bilateral optic atrophy and right hemiparesis. There was no evidence of local disease recurrence at the primary site at her left breast. Brain MRI revealed a solitary left frontal mass with moderate contrast enhancement and perilesional edema [
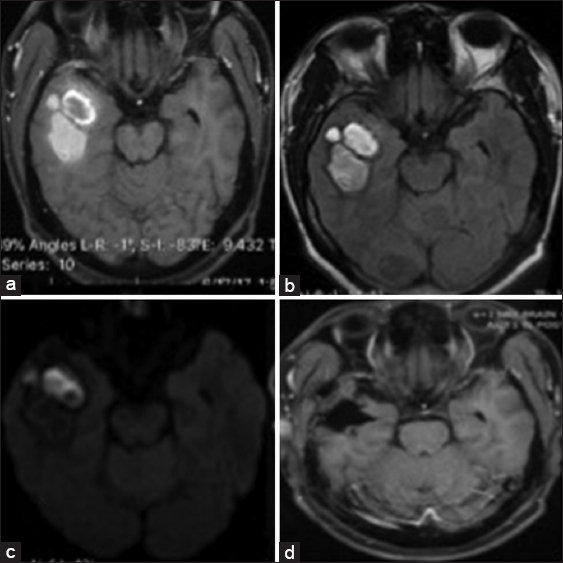
The second patient was a 33-year-old male, who presented with a 3-month history of headache. Prior to presentation, he reported an episode of alteration in the level of consciousness, from which he fully recovered. He was otherwise neurologically intact. Brain MRI revealed a mixed intensity right temporopolar mass with a cystic anterior portion showing peripheral enhancement [
RESULTS
The operative time was about 4 h in each case. Patients were admitted to a postoperative care unit and observed for about an hour before transfer to the neurosurgical ward, as the hospital does not presently have a daycare admission unit. The patients were observed postoperatively for a minimum of 6 h before discharge home in the postoperative unit using standard neurosurgical protocol. They were both discharged on analgesics and steroid, which was tapered off after review on the 1st day postsurgery. The first patient had a postoperative computed tomography (CT) scan done after being discharged home on the 1st day postoperative (due to hospital-related issues). She was clinically and neurologically stable, with no new postoperative deficits and an unchanged KPS at discharge. There was a financial constraint to do a postoperative MRI, which also delayed the start of radiation therapy to the tumor bed and whole brain radiation therapy. Review at the neurosurgery clinic, 6-week postoperative, showed no new neurologic deficits. The second patient had a postoperative CT scan before leaving the hospital as well as a follow-up MRI 8-month postsurgery, which did not show any evidence of tumor recurrence. He did not have postoperative radiation or chemotherapy.
Both patients expressed satisfaction with the awake craniotomy and the process of being discharged on the same day of surgery.
DISCUSSION
One of the fundamentals of elective brain tumor craniotomy is the stratification of patients between those requiring admission to the ICU postoperatively and those that do not. Admission into the ICU following brain tumor surgery allows for close monitoring, early detection of complications, and prompt intervention. Setting up and maintaining neuro-ICUs, however, are rare in developing countries and expensive,[
There are valid concerns regarding the potential development of neurologic and/or nonneurologic complications following elective craniotomies for brain tumors. Neurologic complications include development of new cranial nerve or motor deficits, language problems, aphasia, seizures, and deterioration in the level of consciousness. These complications are often secondary to intraoperative neural injury, intracranial hematoma (particularly in the tumor bed and from extra-axial hematomas), and brain swelling. The peak of the occurrence of postoperative neurologic complications has generally being alluded to occur within the first 6 h after surgery, particularly in cases of postoperative hematoma following supratentorial surgeries for brain tumors.[
The understanding that most significant complications following craniotomy for brain tumors usually occur in the first 6-h postsurgery served as the key motivation not only to discharge patients from the ICU to a ward level setting but also allowed a change in practice toward an early discharge from the hospital. This essentially made it possible to prepare for patients being discharged as early as postoperative day 1 or 2.[
Early success with this change in practice has moved brain surgery toward an already established process of outpatient procedures in other specialties/subspecialties.[
Aside concerns of immediate deterioration postoperatively, any prolonged hospitalization is usually prophylactic and often not therapeutic in, though it is acknowledged that a small percentage of patients may experience delayed deterioration from the hematoma. Such late complications may occur from extra-axial hemorrhage or bleeding into the resection site, cerebral edema, or electrolyte derangement. The big question, therefore, remains: “should we continue to keep patients in the hospital mainly for precautionary reasons?”
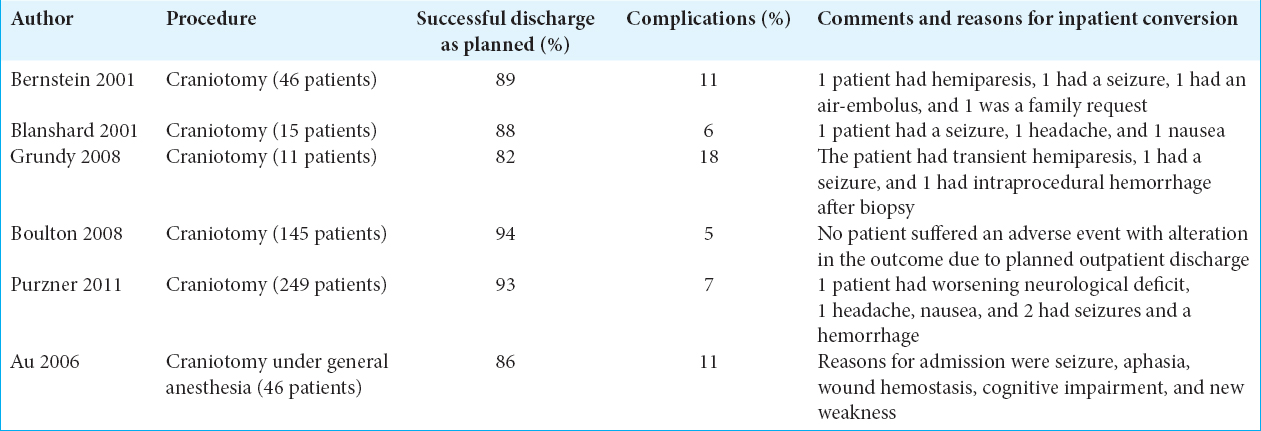
The leap to perform outpatient craniotomies for brain tumors has been mainly championed by the Toronto group of Bernstein is the most senior author of the paper and has been the champion of the procedure not the first author of the paper.[
A more recent report included 44 further cases, which were conducted under a day surgery protocol. In this study, craniotomy for brain tumor was done under general anesthesia and successfully completed in 38/44 patients (86%).[
Our initial experience reported here is, to the best of our knowledge, the first to be documented in any low- or medium-income economy such as sub-Saharan Africa. We elected to perform surgery in our patients employing awake procedures, though our resections were done without intraoperative brain mapping. Such added high-performance technology can be utilized in more “customized” hospital settings, which are more readily available in developed countries. We are aware that while intraoperative brain mapping further maximizes the advantages of awake craniotomy, its other conveniences include avoidance of the negative effects of general anesthesia; shorter hospital stay favoring same day discharge, which was our goal.[
The reports from the Toronto and Southampton groups have involved a substantial number of patients; yet, these studies have failed to expose any significant difference in complication rates in their outpatient craniotomy cohort compared with those patients who were admitted to the inpatient ward,[
None of the two patients in our present report required readmission or developed a new neurologic deficit. This is important when you consider that high mortality rates have been reported in neurosurgical procedures in studies from across Africa.[
Some of the other arguments put forward in favor of outpatient craniotomy for brain tumor include (i) avoidance of overnight hospital stay (=cost reduction) and (ii) the comfort of enjoying one’s privacy at home.[
Enjoying the comfort of home during postsurgical care certainly depends on the access to proper infrastructure and reliable community services; its relevance becomes more apparent in low-income economies where the living conditions may not be suitable or convenient for this type of postoperative care.[
CONCLUSION
We have discussed the practicality, safety, and cost-effectiveness of awake outpatient craniotomy for brain tumors in two carefully selected patients, in a resource-challenged environment. Further prospective studies in similar settings but involving larger groups of patients are warranted and encouraged.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Au K, Bharadwaj S, Venkatraghavan L, Bernstein M. Outpatient brain tumor craniotomy under general anesthesia. J Neurosurg. 2016. 125: 1130-5
2. [ Last accessed on 2018 Sep]. Available from: http://www.databank.worldbank.org/data/download/poverty/33EF03BB-9722-4AE2-ABC7-AA2972D68AFE/Archives -2018/Global_POVEQ_NGA.pdf.
3. Balogun JA, Chernov MF, Kesari S, McCutcheon IE.editors. Surgery of Intracranial Gliomas in Children. Intracranial Gliomas Part I-Surgery. Basel: Karger; 2018. p. 204-17
4. Beauregard CL, Friedman WA. Routine use of postoperative ICU care for elective craniotomy:A cost-benefit analysis. Surg Neurol. 2003. 60: 483-9
5. Bernstein M. Outpatient craniotomy for brain tumor:A pilot feasibility study in 46 patients. Can J Neurol Sci. 2001. 28: 120-4
6. Biccard BM, Madiba TE, Kluyts HL, Munlemvo DM, Madzimbamuto FD, Basenero A. Perioperative patient outcomes in the African surgical outcomes study:A 7-day prospective observational cohort study. Lancet. 2018. 391: 1589-98
7. Boulton M, Bernstein M. Outpatient brain tumor surgery:Innovation in surgical neurooncology. J Neurosurg. 2008. 108: 649-54
8. Bramall A, Djimbaye H, Tolessa C, Biluts H, Abebe M, Bernstein M. Attitudes toward neurosurgery in a low-income country:A qualitative study. World Neurosurg. 2014. 82: 560-6
9. Brown T, Shah AH, Bregy A, Shah NH, Thambuswamy M, Barbarite E. Awake craniotomy for brain tumor resection:The rule rather than the exception?. J Neurosurg Anesthesiol. 2013. 25: 240-7
10. Bui JQ, Mendis RL, van Gelder JM, Sheridan MM, Wright KM, Jaeger M. Is postoperative intensive care unit admission a prerequisite for elective craniotomy?. J Neurosurg. 2011. 115: 1236-41
11. Carrabba G, Venkatraghavan L, Bernstein M. Day surgery awake craniotomy for removing brain tumours:Technical note describing a simple protocol. Minim Invasive Neurosurg. 2008. 51: 208-10
12. D'Amico RS, Kennedy BC, Bruce JN. Neurosurgical oncology:Advances in operative technologies and adjuncts. J Neurooncol. 2014. 119: 451-63
13. Dewan MC, Rattani A, Fieggen G, Arraez MA, Servadei F, Boop FA. Global neurosurgery:The current capacity and deficit in the provision of essential neurosurgical care. Executive summary of the global neurosurgery initiative at the program in global surgery and social change. J Neurosurg. 2018. 130: 1055-1064
14. Gennari A, Mazas S, Coudert P, Gille O, Vital JM. Outpatient anterior cervical discectomy:A french study and literature review. Orthop Traumatol Surg Res. 2018. 104: 581-4
15. Gignoux B, Blanchet MC, Lanz T, Vulliez A, Saffarini M, Bothorel H. Should ambulatory appendectomy become the standard treatment for acute appendicitis?. World J Emerg Surg. 2018. 13: 28-
16. Goettel N, Chui J, Venkatraghavan L, Tymianski M, Manninen PH. Day surgery craniotomy for unruptured cerebral aneurysms:A single center experience. J Neurosurg Anesthesiol. 2014. 26: 60-4
17. Grundy PL, Weidmann C, Bernstein M. Day-case neurosurgery for brain tumours:The early united kingdom experience. Br J Neurosurg. 2008. 22: 360-7
18. Kelly MP, Calkins TE, Culvern C, Kogan M, Della Valle CJ. Inpatient versus outpatient hip and knee arthroplasty:Which has higher patient satisfaction?. J Arthroplasty. 2018. 33: 3402-6
19. Kerimian M, Bastier PL, Reville N, Fierens S, de Gabory L.editors. Feasibility Study of Bilateral Radical Ethmoidectomy in Ambulatory Surgery. European Annals of Otorhinolaryngology. Head and Neck Diseases in Press; 2018. p.
20. Khu KJ, Doglietto F, Radovanovic I, Taleb F, Mendelsohn D, Zadeh G. Patients'perceptions of awake and outpatient craniotomy for brain tumor:A qualitative study. J Neurosurg. 2010. 112: 1056-60
21. Lonjaret L, Guyonnet M, Berard E, Vironneau M, Peres F, Sacrista S. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. 2017. 36: 213-8
22. Ma R, Livermore LJ, Plaha P. Fast track recovery program after endoscopic and awake intraparenchymal brain tumor surgery. World Neurosurg. 2016. 93: 246-52
23. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression:A prospective study of 1003 patients. Neurosurgery. 2011. 69: 119-26
24. Quimby AE, Shamy MC, Rothwell DM, Liu EY, Dowlatshahi D, Stotts G. Anovel neuroscience intermediate-level care unit model:Retrospective analysis of impact on patient flow and safety. Neurohospitalist. 2017. 7: 83-90
25. Rhondali O, Genty C, Halle C, Gardellin M, Ollinet C, Oddoux M. Do patients still require admission to an intensive care unit after elective craniotomy for brain surgery?. J Neurosurg Anesthesiol. 2011. 23: 118-23
26. Sader E, Yee P, Hodaie M. Assessing barriers to neurosurgical care in Sub-Saharan Africa:The role of resources and infrastructure. World Neurosurg. 2017. 98: 682-8000
27. Serletis D, Bernstein M. Prospective study of awake craniotomy used routinely and nonselectively for supratentorial tumors. J Neurosurg. 2007. 107: 1-6
28. Sughrue ME, Bonney PA, Choi L, Teo C. Early discharge after surgery for intra-axial brain tumors. World Neurosurg. 2015. 84: 505-10
29. Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors:A prospective trial of 200 cases. J Neurosurg. 1999. 90: 35-41
30. Taylor WA, Thomas NW, Wellings JA, Bell BA. Timing of postoperative intracranial hematoma development and implications for the best use of neurosurgical intensive care. J Neurosurg. 1995. 82: 48-50
31. Thomas JG, Gadgil N, Samson SL, Takashima M, Yoshor D. Prospective trial of a short hospital stay protocol after endoscopic endonasal pituitary adenoma surgery. World Neurosurg. 2014. 81: 576-83
32. Turel MK, Bernstein M. Is outpatient brain tumor surgery feasible in India?. Neurol India. 2016. 64: 886-95
33. Turel MK, Bernstein M. Outpatient neurosurgery. Expert Rev Neurother. 2016. 16: 425-36
34. Uche EO, Ezomike UO, Chukwu JC, Ituen MA. Intensive care unit admissions in federal medical centre umuahia South East Nigeria. Niger J Med. 2012. 21: 70-3
35. Vukoja M, Riviello E, Gavrilovic S, Adhikari NK, Kashyap R, Bhagwanjee S. Asurvey on critical care resources and practices in low and middle-income countries. Glob Heart. 2014. 9: 337-420
36. Wendlandt B, Bice T, Carson S, Chang L. Intermediate care units:A survey of organization practices across the united states. J Intensive Care Med. 2018. p.
Commentary
Georges Sinclair- Department of Neurosurgery, Bezmialem Vakif University Hospital, Istanbul, Turkey
This two-case study describes the complex reality of oncology patients requiring neurosurgical intervention in a sub-Saharan, resource-restrained environment. The authors have delicately proposed a patient-focused, cost-effective surgical approach aimed at reducing post-operative inpatient care while providing optimal neurological outcome and sustained quality of life. A potential landmark in neurosurgical care. Prospective studies with large-scale settings are encouraged and warranted.