- Department of Neurosurgery, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
- Department of Anatomical Pathology, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
- Department of Urology, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Correspondence Address:
Asra Al Fauzi, Department of Neurosurgery, Universitas Airlangga – Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
DOI:10.25259/SNI_598_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kevin Ariel Tiopan Simanjuntak1, Asra Al Fauzi1, Ayu Yoniko Christi1, Perthdyatama Syifaq Budiono1, Rahadian Indarto Susilo1, Irwan Barlian Immadoel Haq1, Nur Setiawan Suroto1, Dyah Fauziah2, Wahjoe Djatisoesanto3. Clear-cell renal cell carcinoma and glioblastoma multiforme coexistence: Double primary malignancy, does it have a causal relationship?. 12-Aug-2022;13:361
How to cite this URL: Kevin Ariel Tiopan Simanjuntak1, Asra Al Fauzi1, Ayu Yoniko Christi1, Perthdyatama Syifaq Budiono1, Rahadian Indarto Susilo1, Irwan Barlian Immadoel Haq1, Nur Setiawan Suroto1, Dyah Fauziah2, Wahjoe Djatisoesanto3. Clear-cell renal cell carcinoma and glioblastoma multiforme coexistence: Double primary malignancy, does it have a causal relationship?. 12-Aug-2022;13:361. Available from: https://surgicalneurologyint.com/surgicalint-articles/11784/
Abstract
Background: Multiple primary malignancies (MPMs), especially coexistence of renal cell carcinoma (RCC) and glioblastoma multiforme (GBM), are rare. The most likely clinical diagnosis in patient with tumor in another organ is metastatic brain tumor. Although GBM is the most common brain tumor, it is rarely coexistent with other malignancies.
Case Description: A 64-year-old female presented with headache and dizziness, along with abdominal pain for 2 weeks before being admitted. The abdominal computed tomography (CT) scan showed a kidney tumor. The patient developed left hemiplegia, and the brain CT scan showed an intracranial tumor. The patient suggested for radical nephrectomy and craniotomy tumor removal. Histopathology of the kidney and brain tumor revealed two different features, which showed RCC and GBM. Immunohistochemistry result confirmed the diagnosis of GBM and IDH1 wild type; coexistent with clear cell RCC.
Conclusion: The coexistence of carcinoma and glioma should be regarded as coincidental cases if it did not accomplish the criteria for tumor-to-tumor metastasis or proven to be a genetic syndrome. This case report provides an addition to the literature about double primary malignancy in a single patient. More studies are needed to confirm whether they have causal relationship or merely coincidental findings.
Keywords: Case report, Coexisting malignancy, Glioblastoma multiforme, Malignancy, Renal-cell carcinoma
INTRODUCTION
Multiple primary malignancies (MPMs), especially renal cell carcinoma (RCC) and glioblastoma multiforme (GBM) coexistence, are rare. About 90% of kidney cancers are diagnosed with RCC which originated from renal epithelium.[
CASE PRESENTATION
Clinical history
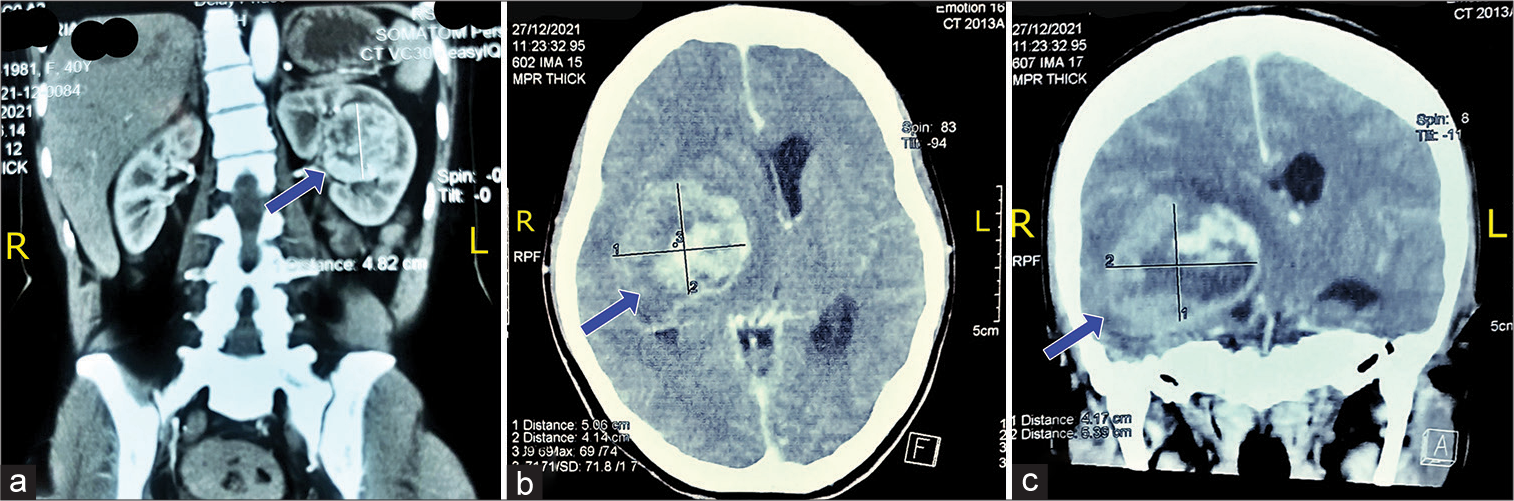
A 64-year-old Asian woman presented with a headache and dizziness along with the left extremities hemiparesis for 3 days before being admitted to the hospital. In depth historical taking, the patient was admitted to another hospital 2 weeks earlier with a headache and dizziness along with abdominal pain. Patient was taken for computed tomography (CT) scan of the abdominal and was diagnosed with kidney tumor. The patient was suggested for nephrotomy and biopsy; however, the patient refused the surgery. The patient was discharged after the general conditions improved. Eight days later, the patient was readmitted to hospital with a headache, dizziness, and severe abdominal pain along with pain in the extremities. Given the patient’s conditions, she was undertaken for inpatient care. A couple days later, the patient developed severe headache and left-sided extremities hemiparesis as well as a decreasing consciousness with Glasgow Coma Scale (GCS) E1V2M4. Following the neurologist suggestion, the brain CT scan was subsequently performed [
Physical examination and radiology findings
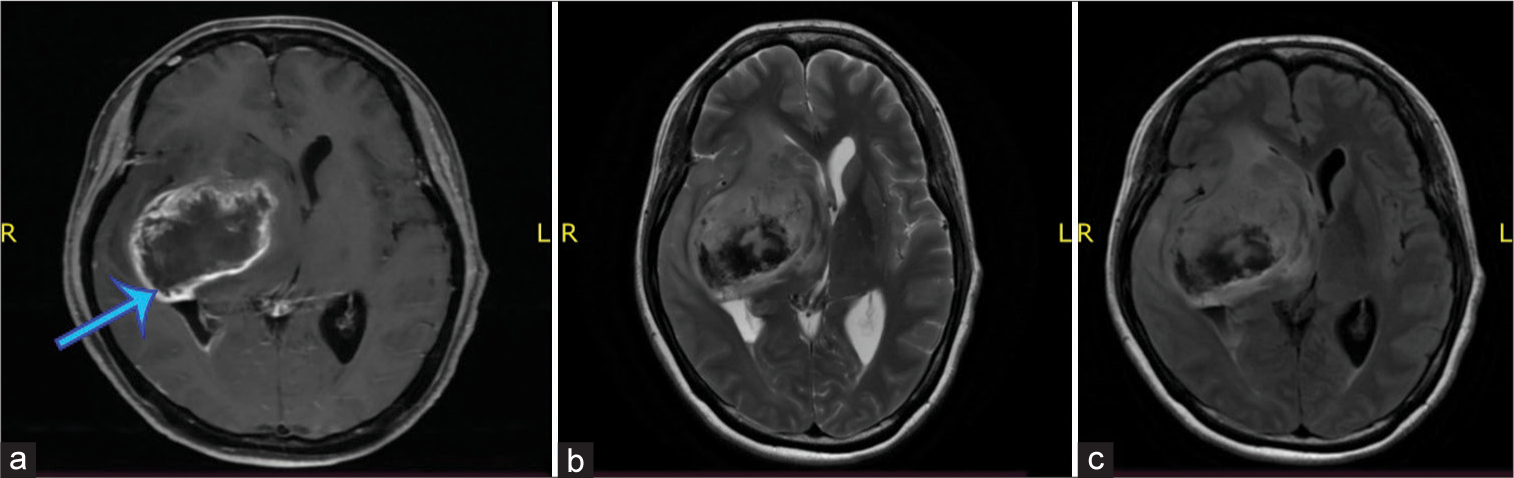
On the initial examination, the patient was alert with GCS of 15. Her blood pressure was 136/85 mmHg which pulse was regular and strong counted in 65 times/min. The patient’s respiratory was 20 times/min and was afebrile. The patient was examined for pain scale with numeric rating scale and according to the patient, the pain score within the scale was 4 (moderate pain). Neurological examination showed diminished muscle strength with grades 2/5 for all muscle groups on the left extremities. The brain magnetic resonance imaging revealed irregular enhancing mass with intra tumoral hemorrhage on deep temporal lobe involving right basal ganglia with 6.3 × 4.2 × 5.2 cm size and adjacent edema suggesting hemorrhagic intracranial metastasis [
Figure 2:
Axial view of brain magnetic resonance imaging, T1 section with contrast (a) showed an irregular peripheral enhancing mass (blue arrow), T2-section without contrast (b), and T1-Flair section without contrast (c) showed a mass with intratumoral hemorrhage, on the deep temporal lobe involving the right basal ganglia with adjacent edema.
Postoperative course
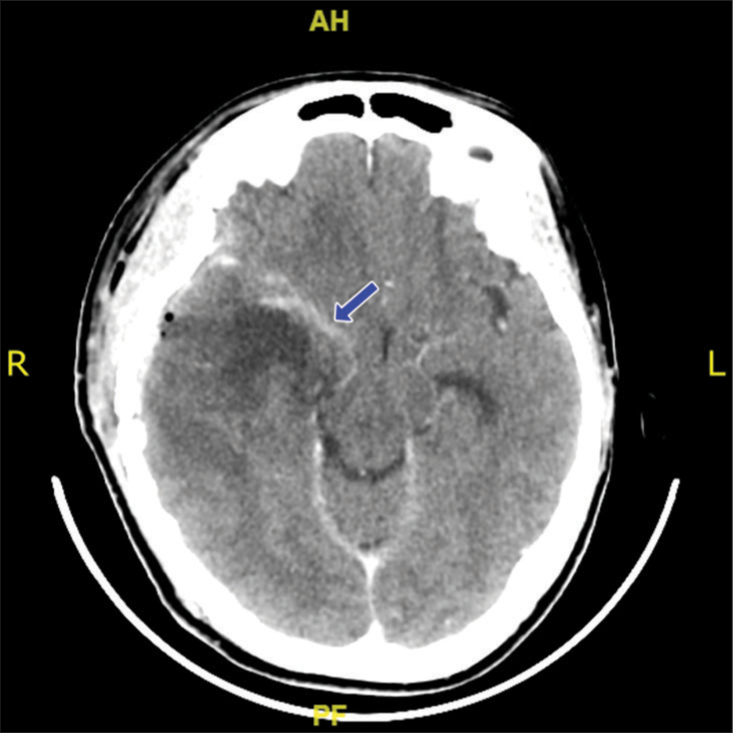
Postoperatively, the patient was admitted to intensive care unit after surgery and extubated on day 6. The brain CT scan evaluation was performed and showed residual tumor with decreased midline shift [
Histopathological report
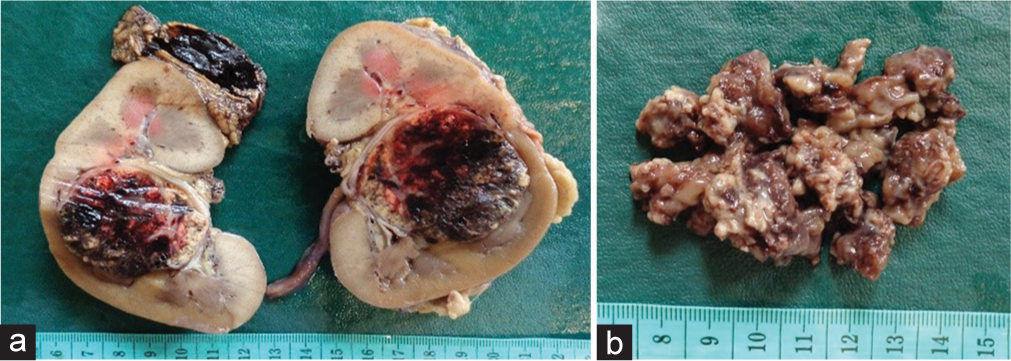
Pathology examination of the left nephrectomy specimen revealed a tumor located at mid pole, measuring 4.5 × 4.5 × 4 cm. Tumor was solid and friable, with yellow and brown in color [
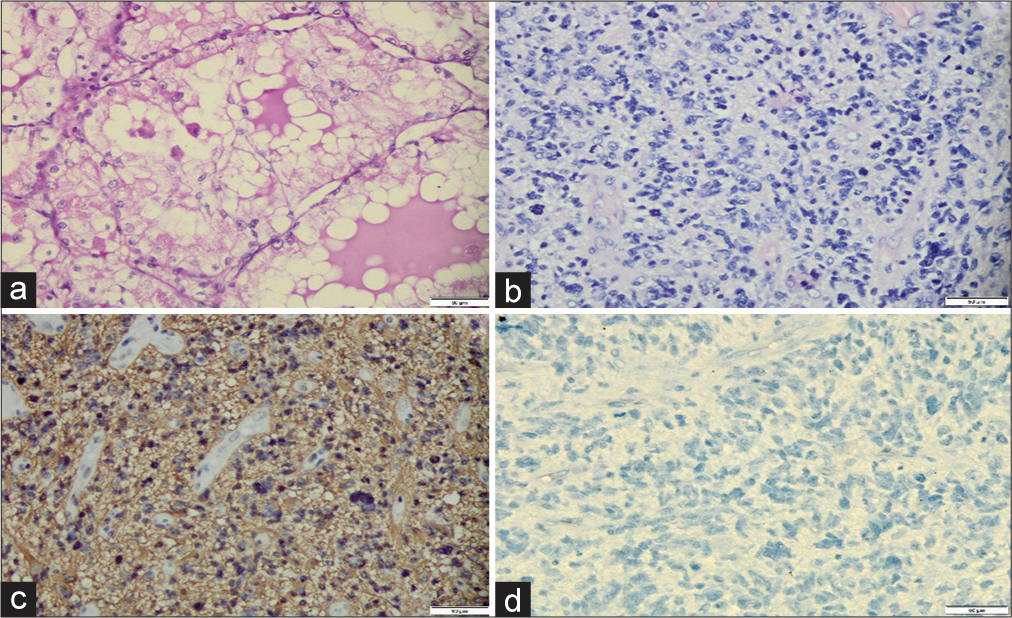
Pathology examination of brain tumor revealed cellular tumor tissue with necrosis and hemorrhages. The tumor cells showed round to oval shaped nuclei, marked pleomorphic, coarse chromatin, with high mitotic activity.
Immunohistochemistry of brain tumor was positive for glial fibrillary acidic protein (GFAP) and ATRX; yet negative for pancytokeratin, CD10, p53, and IDH1 R132H. The final conclusion was glioblastoma, IDH1 wild type, the The World Health Organization (WHO) Grade 4; Coexistent with Clear Cell RCC in the left kidney, and the WHO/International society of urological pathology (ISUP) Grade 2, Stage I [
Figure 5:
Pathology examination of tumor in the left kidney and the brain. (a) The left kidney tumor showed tumor cells displaying rounded nuclei with mild pleomorphism, abundant, and clear cytoplasm, forming a lobular pattern with delicate fibrovascular septa (H&E, ×400). (b) The brain tumor showed tumor cells with rounded to oval-shaped nuclei, coarse chromatin, and brisk mitosis (H&E, ×400). (c) Immunohistochemistry of the brain tumor was positive for GFAP (×200). (d) The brain tumor showed negative for IDH1 R132 H (×200).
DISCUSSION
Coexistence of two malignant tumors is a very rare occurrence especially a report on simultaneous GBM existence with RCC case.[
The incidence rate of MPMs itself is rare, as the consequence, case report on MPMs is also rare. A literature review on patients diagnosed with cancer concluded that the prevalence of MPMs was 0.73% and 11.7%, but MPMs prevalence can be different between one ethnicity to another ethnicity.[
Based on the histology characteristic and tumor formations, meningioma and clear cell RCC exhibit opposing differentiation; it is the same as another malignancy, that is, malignant glioma which is commonly undetected because it has heterogeneous composition of the neoplastic tissue.[
Dobbing and Campbell et al. defined tumor-to-tumor metastasis using rigorous standards: The presence of two or more distinct tumors, the recipient tumor must be a true neoplasm, and there must be an actual metastatic deposit within the neoplastic tissue of the recipient tumor.[
Since GFAP is positive in glioma cells and normal brain tissue, this can be applied to differentiate between glioblastoma and RCC. In this case, the brain tumor showed different morphological features with tumor of the kidney. However, immunohistochemistry was performed to confirm that the brain tumor and the kidney tumor were two different entities. Immunohistochemistry of the brain tumor showed negative for pancytokeratin and CD10, yet positive for GFAP. The morphology and immunohistochemistry findings supported a diagnosis of glioblastoma. Subsequent immunohistochemistry with IDH1 R132H, ATRX, and p53 established a diagnosis of glioblastoma and IDH wild type.
GFAP is the mark of intermediate filament protein in astrocyte in the central nervous system (CNS). Hence, to differ between metastatic cell carcinoma and glioma, we can use GFAP as the immunohistochemistry examination.[
A study by Budka regarding GFAP as a biomarker on glioma showed that renal carcinoma metastatic to the brain had a strong reaction in moderate cells for anti-GFAP. This result was affected by two possibilities such as immunoreactive cells might represent reactive astroglia and dense gliosis in suspected vicinity.[
MPMs are always associated with genetic syndrome. Several genetic syndromes associated with CNS tumors especially GBM are TS, LFS, and von-Hippel Lindau (VHL) syndrome. TS is a genetic syndrome for MPM with association of primary tumors of CNS and two different forms of colorectal polyp, while TS is divided into two types; TS Type I is characterized with the presence of glial tumors with few colonic polyps, while TS Type II is characterized with thousands of colonic polyps and increased risk of medulloblastoma.[
Another genetic syndrome associated with CNS tumor is LFS. LFS is autosomal dominant disease caused by germline mutation in gene locus 17p13 which encode Tumor Protein 53 (TP53) gene, so LFS is having a lifelong increased risk of developing multiple tumors especially intracranial malignancy such as GBM.[
In the study attempted by Tajika et al., it was reported that in the epithelial line of the glioma from bronchial carcinoma showed immunoreactivity for cytokeratins; it had acinar and papillary patterns which were demarcated on the surrounding glioma tissue.[
CONCLUSION
The coexistence of carcinoma and glioma in a single patient is rare. If the coexistence of both tumors did not accomplish the criteria for tumor-to-tumor metastasis and was not proven as a genetic syndrome, it should be regarded as coincidental finding, as seen in our case. This case report provides an addition to the literature about double primary malignancy in a single patient. However, more studies are needed to confirm whether they have causal relationship or merely coincidental findings.
Ethical approval
This is a case report; therefore, it did not require ethical approval from the ethics committee. However, we obtained permission from each patient’s parents to publish their data.
Consent
Written informed consent was obtained from each patient’s family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
This case report is not eligible for obtaining a research registry since it only contains a report of a known entity with no new surgical or medical interventions.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bao Q, Yu S, Yu X. Collision tumor of meningioma and metastatic renal clear cell carcinoma: A case report. Br J Neurosurg. 2020. p. 1-3
2. Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 2017. 24: 3002-9
3. Budka H. Non-glial specificities of immunocytochemistry for the glial fibrillary acidic protein (GFAP) Triple expression of GFAP, vimentin and cytokeratins in papillary meningioma and metastasizing renal carcinoma. Acta Neuropathol. 1986. 72: 43-54
4. Campbell LV, Gilbert E, Chamberlain CR, Watne AL. Metastases of cancer to cancer. Cancer. 1968. 22: 635-43
5. Crisman CM, Patel AR, Winston G, Brennan CW, Tabar V, Moss NS. Clinical outcomes in patients with renal cell carcinoma metastases to the choroid plexus. World Neurosurg. 2020. 140: e7-13
6. Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: Case report and a comprehensive review of the literature. Am J Clin Oncol. 2003. 26: 79-83
7. Dipro S, Al-Otaibi F, Alzahrani A, Ulhaq A, Al Shail E. Turcot syndrome: A synchronous clinical presentation of glioblastoma multiforme and adenocarcinoma of the colon. Case Rep Oncol Med. 2012. 2012: 720273
8. Dobbing J. Cancer to cancer. Guys Hosp Rep. 1958. 107: 60-5
9. Franke FE, Altmannsberger M, Schachenmayr W. Metastasis of renal carcinoma colliding with glioblastoma. Carcinoma to glioma: An event only rarely detected. Acta Neuropathol. 1990. 80: 448-52
10. Gray RE, Harris GT. Renal cell carcinoma: Diagnosis and management. Am Fam Physician. 2019. 99: 179-84
11. Hol EM, Pekny M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol. 2015. 32: 121-30
12. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M. Renal cell carcinoma. Nat Rev Dis Primers. 2018. 3: 1117-27
13. Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015. 10: e0125754
14. Luciani A, Ascione G, Marussi D, Oldani S, Caldiera S, Bozzoni S. Clinical analysis of multiple primary malignancies in the elderly. Med Oncol. 2009. 26: 27-31
15. Maher ER. Hereditary renal cell carcinoma syndromes: Diagnosis, surveillance and management. World J Urol. 2018. 36: 1891-8
16. Mörk SJ, Rubinstein LJ. Metastatic carcinoma to glioma: A report of three cases with a critical review of the literature. J Neurol Neurosurg Psychiatry. 1988. 51: 256-9
17. Omuro A, De Angelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013. 310: 1842-50
18. Petraki C, Vaslamatzis M, Argyrakos T, Petraki K, Strataki M, Alexopoulos C. Tumor to tumor metastasis: Report of two cases and review of the literature. Int J Surg Pathol. 2003. 11: 127-35
19. Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E. Multiple tumours in survival estimates. Eur J Cancer. 2009. 45: 1080-94
20. Siomin V, Lin JL, Marko NF, Barnett GH, Toms SA, Chao ST. Stereotactic radiosurgical treatment of brain metastases to the choroid plexus. Int J Radiat Oncol Biol Phys. 2011. 80: 1134-42
21. Sun M, De Velasco G, Brastianos PK, Aizer AA, Martin A, Moreira R. The development of brain metastases in patients with renal cell carcinoma: Epidemiologic trends, survival, and clinical risk factors using a population-based cohort. Eur Urol Focus. 2019. 5: 474-81
22. Tajika Y, Reifenberger G, Kiwit JC, Wechsler W. Metastatic adenocarcinoma in cerebral astrocytoma: Clinicopathological and immunohistochemical study with review of the literature. Acta Neurochir (Wien). 1990. 105: 50-5
23. van Asperen JV, Fedorushkova DM, Robe PA, Hol EM. Investigation of glial fibrillary acidic protein (GFAP) in body fluids as a potential biomarker for glioma: A systematic review and meta-analysis. Biomarkers. 2022. 27: 1-12
24. Vijapura C, Aldin ES, Capizzano AA, Policeni B, Sato Y, Moritani T. Genetic syndromes associated with central nervous system tumors. Radiographics. 2017. 37: 258-80
25. Warren S, Gates O. Multiple primary malignant tumors: A survey of the literature and statistical study. Am J Cancer. 1932. 16: 1358-414
26. Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou X. Multiple primary malignant tumors-a clinical analysis of 15,321 patients with malignancies at a single center in China. J Cancer. 2018. 9: 2795-801