- Department of Neurology and Neurosurgery, Montreal Neurological Institute and Hospital, McGill University, Montreal, Quebec, Canada
- Department of Neurosurgery, Kurashiki Central Hospital, Kyoto University, Okayama, Japan
- Division of Neurosurgery, St. Michael's Hospital, University of Toronto, Toronto, Ontario, Canada
- Department of Surgery, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
- Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
- Department of Medicine, Division of Clinical Pharmacology, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
Correspondence Address:
Benjamin W. Y. Lo
Department of Medicine, Division of Clinical Pharmacology, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, Canada
DOI:10.4103/2152-7806.187496
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Y. Lo BW, Fukuda H, Angle M, Teitelbaum J, Macdonald RL, Farrokhyar F, Thabane L, H. Levine MA. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage – Alterations in brain–body interface. Surg Neurol Int 01-Aug-2016;7:
How to cite this URL: Y. Lo BW, Fukuda H, Angle M, Teitelbaum J, Macdonald RL, Farrokhyar F, Thabane L, H. Levine MA. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage – Alterations in brain–body interface. Surg Neurol Int 01-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/clinical-outcome-prediction-aneurysmal-subarachnoid-hemorrhage-alterations-brain-body-interface/
Abstract
Background:Brain–body associations are essential in influencing outcome in patients with ruptured brain aneurysms. Thus far, there is scarce literature on such important relationships.
Methods:The multicenter Tirilazad database (3551 patients) was used to create this clinical outcome prediction model in order to elucidate significant brain–body associations. Traditional binary logistic regression models were used.
Results:Binary logistic regression main effects model included four statistically significant single prognostic variables, namely, neurological grade, age, stroke, and time to surgery. Logistic regression models demonstrated the significance of hypertension and liver disease in development of brain swelling, as well as the negative consequences of seizures in patients with a history of myocardial infarction and post-admission fever worsening neurological outcome.
Conclusions:Using the aforementioned results generated from binary logistic regression models, we can identify potential patients who are in the high risk group of neurological deterioration. Specific therapies can be tailored to prevent these detriments, including treatment of hypertension, seizures, early detection and treatment of myocardial infarction, and prevention of hepatic encephalopathy.
Keywords: Aneurysmal subarachnoid hemorrhage, brain-body interactions, clinical outcome prediction model
INTRODUCTION
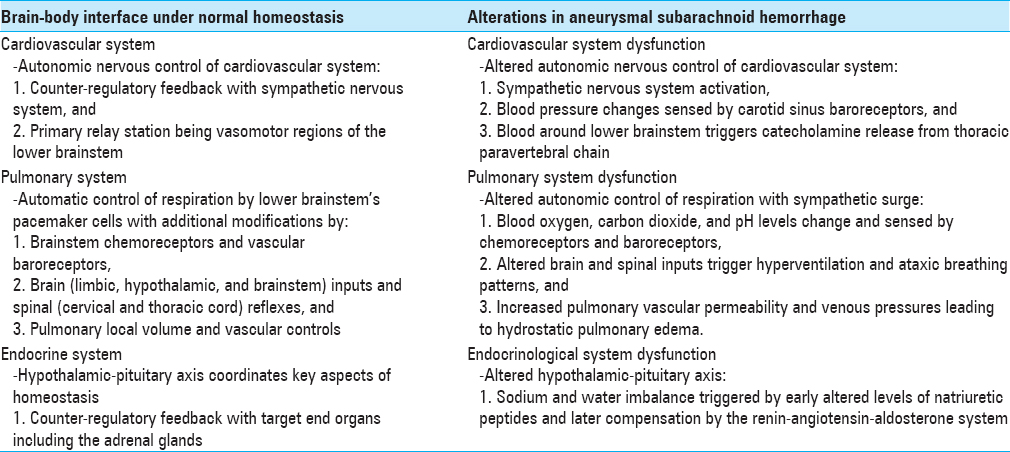
The brain–body interface comprises many physiological interactions which can go awry in disease states. Under conditions of normal homeostasis, brain–body associations are frequently characterized as interactions between the human nervous system and the cardiovascular, respiratory, endocrinological, and gastrointestinal systems.
The brain–body interface
Cardiovascular-nervous system associations
Direct and indirect projections of the autonomic nervous system control the cardiovascular system. These projections act via the parabrachial nucleus of the midbrain and pons. The parabrachial nucleus is a relay station that transmits signals between the cerebral cortex (especially limbic cortex), amygdala, hypothalamic paraventricular nucleus, vasomotor area of the lower brainstem (including the ventrolateral medulla, medullary raphe, nucleus of the solitary tract), and the thoracolumbar interomediolateral gray column of the spinal cord.[
The autonomic nervous system mediates the heart rate (chronotrophy), rate of nervous impulse transmission through the cardiac conductive tissue (dromotropy), and force of contraction (inotropy). Blood vessel diameter and tone are also mediated via the autonomic nervous system.
Counter-regulatory feedback with the sympathetic nervous system predominates. An example of such counter-regulatory feedback is the Cushing reflex. Increase in intracranial pressure from disease states, such as intracerebral hemorrhage, leads to decreased levels of oxygen and increased local levels of hydrogen ions and carbon dioxide around the vasomotor regions of the lower brainstem secondary to anaerobic metabolism. As a counter-regulatory measure, the systemic blood pressure is elevated in an attempt to increase blood flow to the brain. With this increase in blood pressure, heart rate is reflexively decreased via the arterial baroreceptors.[
Other factors also affect the amount of autonomic nervous output from the vasomotor regions of the brainstem, including
Blood oxygen level, Blood carbon dioxide level, Pain stimulus, Chemoreceptors and baroreceptors in the carotid, pulmonary, and aortic vessels sense corresponding changes, Nerve impulse feedback from the lungs secondary to lung inflation and deflation, and Regulatory signals between the cerebral cortex and brainstem.
Respiratory-nervous system associations
Pacemaker cells in the medulla are responsible for the autonomic control of respiration. Their rhythmic discharges are modified by pneumotaxic neurons in the pons, as well as limbic and hypothalamic lesions.[
Impulses from afferent pulmonary vagal fibers with lung inflation and deflation, Spinal reflexes from cervical and thoracic spinal cord to the diaphragm and intercostal muscles, Changes in the cerebrospinal fluid concentrations of carbon dioxide and hydrogen ions sensed by medullary chemoreceptors, and Changes in oxygen tension sensed by baroreceptors in the aorta, cardiac atrium, and ventricle, as well as pulmonary arteries.
Endocrinological-nervous system associations
The hypothalamic-pituitary axis regulates the key aspects of homeostasis, temperature control, and endocrinological functions. This system integrates inputs from both the limbic system and brainstem in order to modulate both emotional and instinctual reactions to changes in the internal milieu.[
Gastrointestinal-nervous system associations
The gastrointestinal autonomic nervous system regulates the central nervous system inputs to the enteric system regarding gut motility and secretory functions. It works in conjunction with local paracrine pathways.[
Aneurysmal subarachnoid hemorrhage and alterations in brain–body associations
Patients with ruptured brain aneurysms and associated subarachnoid hemorrhage (SAH) have a mortality rate of at least 45% in the first month after rupture.[
The Tirilazad database
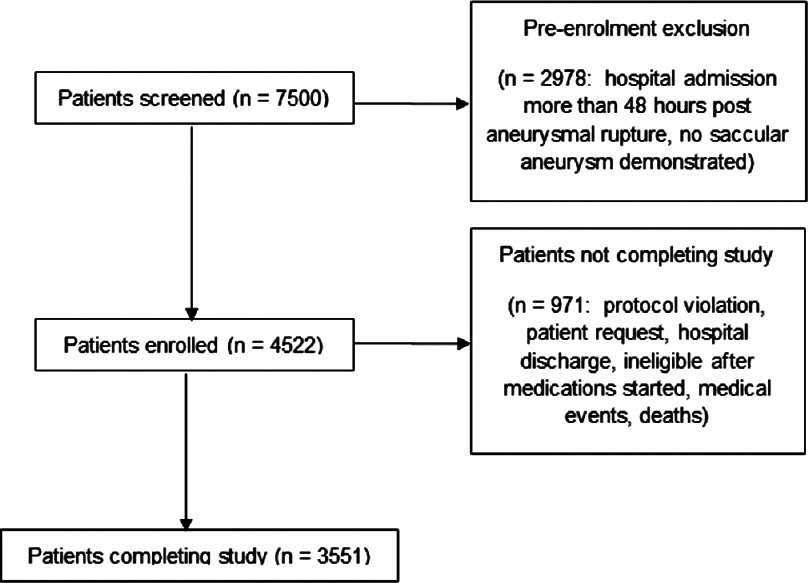
Tirilazad was a 21-aminosteroid compound produced by Pharmacia and Upjohn, Kalamazoo, Michigan. It was originally investigated as a free radical scavenger for the potential treatment of cerebral vasospasm. Between 1991 and 1997, 3551 patients from 171 centers in 22 countries in North America, Europe, Australia, New Zealand, and Africa participated in five randomized, double-blind, placebo-controlled trials of Tirilazad.[
Included patients were adults with evidence of aneurysmal SAH. The patients received either placebo or active agent (in intravenous doses of 0.6, 2.6, or 15 mg/kg/day depending on the trial) from the third to tenth day after onset of SAH. Excluded patients were:
Those with traumatic SAH, Those with infectious mycotic aneurysms, Severe underlying medical illnesses including serious cardiovascular diseases, such as myocardial infarction within the previous 6 months, uncontrolled hypertension, serious cardiac arrthymias, or congestive heart failure, Pregnant or lactating patients, Patients taking corticosteroids or calcium channel blockers other than nimodipine, Known intolerance to calcium channel blockers, and Patients whose aneurysms were treated with Gulielmi or other detachable coils.
Patients in the placebo and treatment arms were otherwise treated similarly. Over 85% of patients underwent surgical clipping, and half of them were operated within the first 48 hours. Baseline demographics in both the arms were balanced in terms of sex, age, number of pre-existing medical conditions, mean time to treatment, mean admission systolic blood pressure, admission neurological grade, ruptured aneurysm location, amount of blood visualized on admission computed tomography (CT) scan (measured by the Fisher scale taking into account thickness of the blood clot with or without intraventricular component). The potential confounder of patient sex was accounted for in statistical analysis in the North American Tirilazad trial where fewer female patients were randomized to the treatment arm.[
Only 1% of patients were lost to follow-up. The primary outcome measure was the patient's Glasgow Outcome Score (GOS) at 3 months after aneurysmal rupture. GOS is a 5-point scale, defined as: 5 – good recovery with normal life activities despite minor deficits, 4 – moderate disability being disabled but independent, 3 – severe disability being conscious but dependent, 2 – persistent vegetative state, and 1 – death.[
Two systematic reviews involving the five Tirilazad trials found no substantial heterogeneity among these trials, and no significant differences between treatment and placebo arms, or among patients who were administered different dosages of Tirilazad regarding the number of patients who died, the number of adverse events, or neurological outcome at 3 months follow-up.[
Objectives
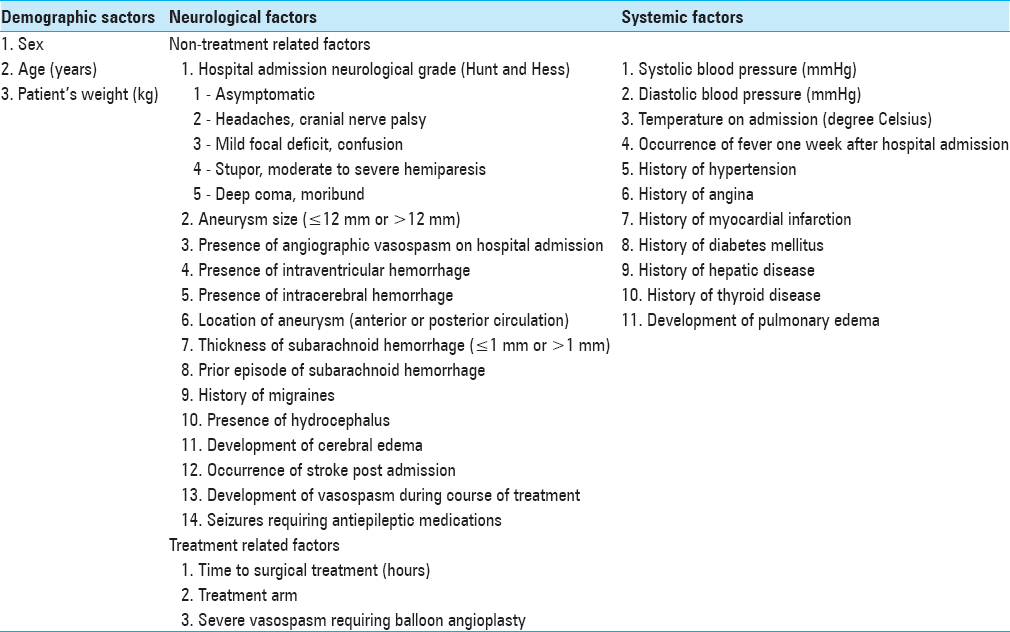
Using data from the placebo and Tirilazad arms of the five clinical trials, we aim to create a clinical outcome prediction model using binary logistic regression and investigate the potential factors for outcome prediction in patients with ruptured brain aneurysms. In particular, we assess the interaction of potential factors and explore the role for possible associations between the brain and other organ systems.
MATERIALS AND METHODS
The Tirilazad database was used to investigate the potential factors for outcome prediction in aneurysmal SAH and to assess interactions of factors between the brain and other organ systems.
The dependent variable used for statistical analysis is the dichotomized GOS at three months, that is, good or poor outcome. Good outcome represents functional independence (GOS 5 or 4). Poor outcome represents functional dependence (GOS 3), persistent vegetative state (GOS 2) or death (GOS 1).[
Using IBM's the Statistical Package for the Social Sciences version 19.0™, independent variables were entered into univariate models in which Chi-square analyses were performed to investigate associations with poor outcome. Two-way interaction terms that included neurologic and systemic prognostic factors were examined [
In the multivariable logistic regression model, the following diagnostics were performed:
Correlation matrix to rule out multicollinearity among predictor variables, Hosmer and Lemeshow test to ensure good data fit to model and model calibration, C statistic and classification to examine model discrimination, and Split-sample analysis (70% training and 30% testing) to examine model generalizability.
RESULTS
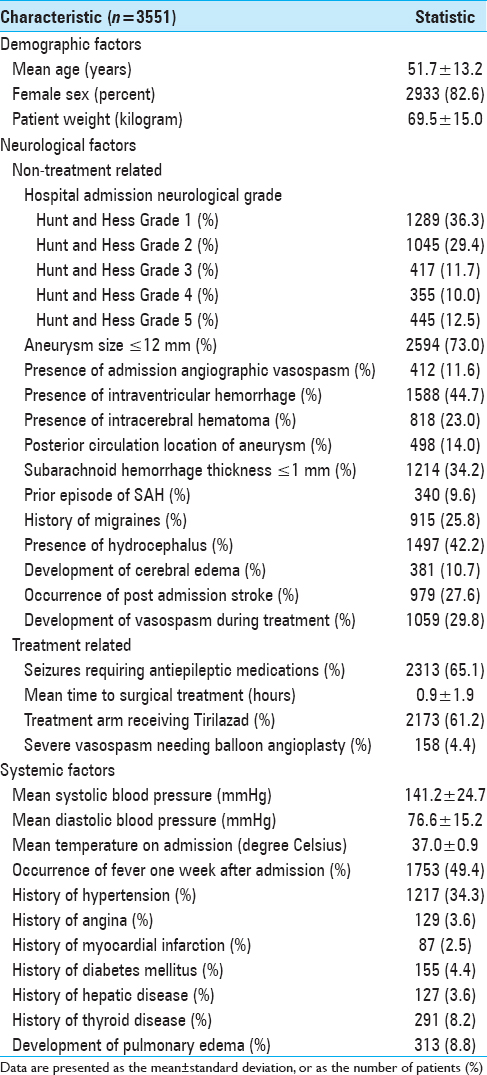
Patient selection and participation in the Tirilazad trials are summarized in
Correlated matrix of the included independent variables was examined to ensure that there were no correlated variables. Variables and interaction terms that reached a probability of 0.10 were entered into the multivariable binary logistic regression model. These variables and interaction terms are summarized in
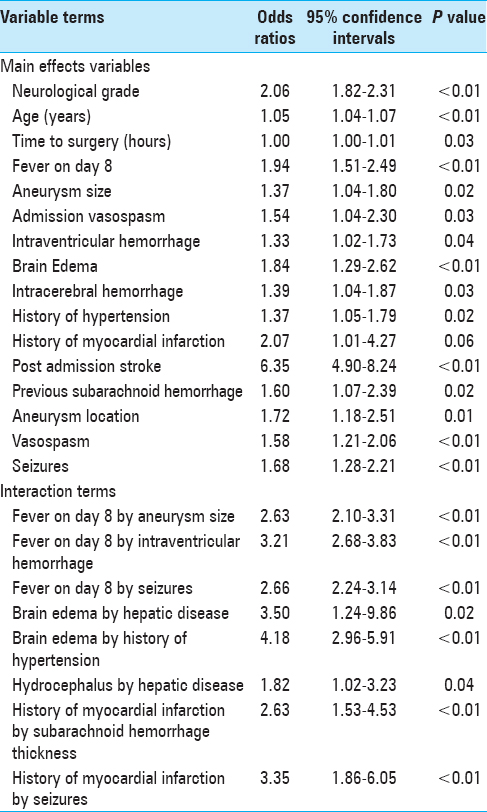
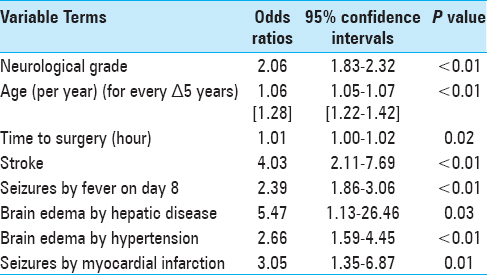
The multivariable binary logistic regression model found four statistically significant single prognostic variables, namely, admission neurological grade, age, time to surgery and post-admission stroke, and four significant interaction terms. These observations are listed as follows:
The odds of poor outcome associated with one unit increase in neurological grade is increased by a factor of 2.06, The odds of poor outcome associated with one unit (every 5 years) increase in age is increased by a factor of 1.28, The odds of poor outcome associated with one unit (every hour) increase in time to surgery is increased by a factor of 1.01, and The odds of poor outcome is increased by a factor of 4.0 when the aneurysmal SAH patient experiences post-hospital admission stroke.
The multivariable binary logistic regression model also found four statistically significant interaction terms. These observations are listed as follows:
The odds of poor outcome is increased by a factor of 2.39 when the aneurysmal SAH patient experiences fever 1 week post-hospital admission and seizures, The odds of poor outcome is increased by a factor of 5.47 when the aneurysmal SAH patient with history of hepatic disease develops cerebral edema, The odds of poor outcome is increased by a factor of 2.66 when the aneurysmal SAH patient with history of hypertension develops cerebral edema, and The odds of poor outcome is increased by a factor of 3.05 when the aneurysmal SAH patient with history of myocardial infarction develops seizures.
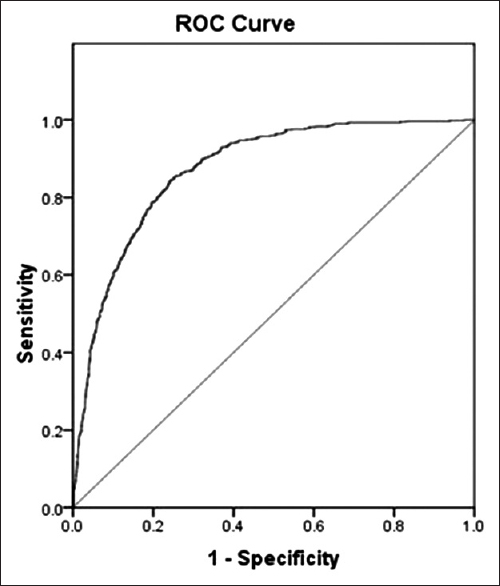
The Omnibus Tests of Model Coefficients indicated that this model was statistically significant (Chi-square = 887.90, P < 0.01). This model showed a large significant reduction in −2 log likelihood (−2 LL from 2240 to 1699, P < 0.01). Nagelkerke R2 statistic indicated that approximately 47% of the variance in poor neurologic outcome could be predicted from the combination of the significant terms. In terms of model discrimination [
Classification table indicated that 84% of subject outcomes were predicted correctly. In terms of model calibration, the Hosmer and Lemeshow test showed a Chi-square of 11.38, P = 0.18, suggesting that the model appeared to fit the data well. Generalizability of model findings was supported by split sample validation (70% training and 30% testing). The following are the findings:
No multicollinearity (standard errors for beta coefficients less than 2), Individual relationship Wald statistic significance less than alpha level of 0.05, and Classification accuracy rate for training sample was 84.5%, whereas classification accuracy rate for testing sample was 82.5%, both satisfying minimum requirement for holdout sample (0.9 × 84.5% = 76%), and Classification accuracy (84.5%) was 1.025 times chance accuracy (82.5%).
DISCUSSION
There has been scarce literature attempting to characterize complex brain–body associations in patients with ruptured brain aneurysms. This analysis included both non-treatment and treatment related prognostic factors, as well as two-way interactions, which described clinically relevant brain–body associations in aneurysmal SAH.
The main effects logistic regression model confirmed the significance of neurological grade, age, stroke, and time to surgery in outcome prognosis in aneurysmal SAH.
This study also demonstrates that the odds of poor neurological outcome is increased by a factor of 4 in aneurysmal SAH patients who develop post-admission strokes (OR: 4.03, 95% CI: 2.11–7.69, P < 0.01). With modern modalities of routine cerebral surveillance imaging over the course of treatment, up to half of aneurysmal SAH patients have observed non-resolving hypodensities and persistent areas of cerebral perfusion deficits, which is consistent with stroke. It is increasingly recognized that cerebral infarction after aneurysmal SAH may occur early after aneurysmal rupture or in a delayed manner. Factors associated with development of cerebral infarction include admission neurological status, treatment related complications, and occurrence of symptomatic vasospasm.[
In addition, this study found that timing to surgical treatment of ruptured aneurysms is significant in determining neurological outcome. This is supported from the observation that shorter time interval to treatment is associated with reduction in aneurysmal rebleeding rates.[
We also make the observation that development of cerebral edema, in the context of history of hypertension and liver disease, has significant impact on neurologic outcome deterioration in aneurysmal SAH.
By itself, development of cerebral edema may predispose the aneurysmal SAH to poor neurological outcome (OR: 1.84, 95% CI: 1.29–2.62, P < 0.01, univariate analysis). Further examination of systemic factors revealed that aneurysmal SAH patients with a history of hypertension and development of cerebral edema have 2.7 fold increased odds of poor neurologic outcome (OR: 2.66, 95% CI 1.59–4.45, P < 0.01). Patients with a history of hypertension are more prone to defective cerebral autoregulation. When cerebral dysautoregulation is present after aneurysmal SAH, brain engorgement can occur as plasma proteins leak from capillaries with increased permeability. Extracellular vasogenic edema may follow as a result of increased hydrostatic pressures, with predilection for posterior cerebral circulation territories.[
This study also makes the novel observation that development of cerebral edema in aneurysmal SAH patients with a history of liver dysfunction markedly increases likelihood for poor outcome (OR: 5.47, 95% CI: 1.13–26.46, P = 0.03). Similar to patients with hypertensive history, patients with chronic liver disease have been shown to have altered cerebral autoregulation and cerebral blood flow with decreased cerebral blood flow in the anterior cingulum and increased blood flow in the basal ganglia and occipital lobes at baseline. In acute states of ruptured cerebral aneurysms, these patients’ blood brain barriers become disrupted with marked increased cerebral blood flow secondary to luxuriant perfusion, thus, predisposing them to the development of vasogenic edema. In addition, cytotoxic osmoregulatory mechanisms are involved whereby astrocytes swell secondary to the toxic effects of ammonia and glutamate. The end result is a vicious cycle of neuronal swelling and death, marked increased in cerebral blood flow (cerebral hyperemia), and cerebral edema.[
Seizures post-aneurysmal SAH increase mortality. The observed incidence of seizures can be as high as 15% in the aneurysmal SAH patient population.[
Finally, our analysis makes the observation that seizures in the setting of history of myocardial infarction increase the odds of poor outcome by a factor of 3.05 (95% CI: 1.35–6.87, P = 0.01). Repetitive autonomic stimulation can occur in the actively seizing aneurysmal SAH patient in a lock step phenomenon, which can trigger the development of cardiac ictal arrthymias.[
Methodological strengths
Our analyses made use of a large multicenter database of aneurysmal SAH patients. Multivariable logistic regression model is a robust statistical technique applicable to this patient cohort because there were: (1) No significant differences in patient outcome (benefit or harm) between placebo and treatment arms, (2) no substantial heterogeneity among different Tirilazad trials, (3) baseline demographics in both arms were balanced, (4) patients in both arms were treated similarly, with statistical analyses accounting for potential confounders, (5) similar percentages of patients experiencing neurological and systemic disabilities in placebo and different treatment groups, and (6) no significant between-center and between-country effects on patient outcome.[
In addition, we investigated both single prognostic factors and explored brain–body interactions. In so doing, several novel observations were found which significantly influence clinical outcome in aneurysmal SAH patients. Furthermore, we examined model discrimination, calibration and validity.
Limitations
This is a retrospective observational study which included heterogeneous clusters of patients from different geographical regions. Patients of various genetic backgrounds may exhibit different clinical responses to treatments such as pharmacokinetic profiles to medications. In addition, the Tirilazad database did not capture important modifiable variables of behaviours including smoking and alcohol consumption. Referral bias is also present because these patients were admitted to and treated at specialized centers with neurosurgical and critical care capabilities.
Future directions
Further studies can be carried out to generalize these observations to other aneurysmal SAH patient populations, including those undergoing coiling.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andreasen TH, Bartek J, Andresen M, Springborg JB, Romner B. Modifiable risk factors for aneurysmal subarachnoid hemorrhage. Stroke. 2013. 44: 3607-12
2. Barrett K, Ganong W.editorsGanong's review of medical physiology. New York: McGraw-Hill Medical; 2010. p.
3. Bederson JAANS Publications Committee. Subarachnoid hemorrhage: Pathophysiology and management. Park Ridge, Ill.: American Association of Neurological Surgeons. 1997. p.
4. Butterworth R. The liver-brain axis in liver failure: Neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013. 10: 522-8
5. Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004. 4: 43-6
6. Detry O, De Roover A, Honore P, Meurisse M. Brain edema and intracranial hypertension in fulminant hepatic failure: Pathophysiology and management. World J Gastroenterol. 2006. 12: 7405-12
7. Dimopoulou I, Kouyialis AT, Tzanella M, Armaganidis A, Thalassinos N, Sakas DE. High incidence of neuroendocrine dysfunction in long-term survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2004. 35: 2884-9
8. Dooley J, Sherlock S.editorsSherlock's diseases of the liver and biliary system. Chichester, West Sussex; Hoboken, NJ: Wiley-Blackwell; 2011. p.
9. Dube CM, Brewster AL, Baram TZ. Febrile seizures: Mechanisms and relationship to epilepsy. Brain and Dev. 2009. 31: 366-71
10. Dumont T, Rughani A, Silver J, Tranmer BI. Diabetes mellitus increases risk of vasospasm following aneurysmal subarachnoid hemorrhage independent of glycemic control. Neurocrit Care. 2009. 11: 183-9
11. Guyton A, Hall J.editorsTextbook of medical physiology. Philadelphia: Saunders; 2000. p.
12. Haley EC, Kassell NF, Alves WM, Weir BK, Hansen CA. Phase II trial of tirilazad in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1995. 82: 786-90
13. Haley EC, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: A cooperative study in North America. J Neurosurg. 1997. 86: 467-74
14. Horiuchi T, Tanaka Y, Hongo K. Surgical treatment for aneurysmal subarachnoid hemorrhage in the 8th and 9th decades of life. Neurosurgery. 2005. 56: 469-75
15. Hosmer D, Lemeshow S, Sturdivant R.editorsApplied logistic regression. Hoboken, NH: Wiley; 2013. p.
16. Ilodigwe D, Murray GD, Kassell NF, Torner J, Kerr RS, Molyneux AJ. Sliding dichotomy compared with fixed dichotomization of ordinal outcome scales in subarachnoid hemorrhage trials. J Neurosurg. 2013. 118: 3-12
17. Jang Y, Ilodigwe D, Macdonald R. Metaanalysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009. 10: 141-7
18. Jansen K, Lagae L. Cardiac changes in epilepsy. Seizure. 2010. 19: 455-60
19. Jennett B, Bond M. Assessment of outcome after severe brain damage: A practical scale. Lancet. 1975. 305: 480-4
20. Kang J, Shen W, Macdonald R. Why does fever trigger febrile seizures?. J Neurosci. 2006. 26: 2590-7
21. Kassell N, Haley E, Apperson-Hansen C, Alves W. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: A cooperative study in Europe, Australia and New Zealand. J Neurosurg. 1996. 84: 221-8
22. Kim W, Neal L, Hoh B. Risk factors, incidence and effect of cardiac failure and myocardial infarction in aneurysmal subarachnoid hemorrhage patients. Neurosurgery. 2013. 73: 450-7
23. Kumar A, Brown R, Dhar R, Sampson T, Derdeyn CP, Moran CJ. Early vs delayed cerebral infarction after aneurysm repair after subarachnoid hemorrhage. Neurosurgery. 2013. 73: 617-23
24. Lannes M, Teitelbaum J, del Pilar Cortes M, Cardoso M, Angle M. Milrinone and homeostasis to treat cerebral vasospasm associated with subarachnoid hemorrhage: The Montreal Neurological Hospital protocol. Neurocrit Care. 2012. 16: 354-62
25. Lanzino G, Kassel N. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999. 90: 1018-24
26. Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand and South Africa. J Neurosurg. 1999. 90: 1011-7
27. Layon A, Gabrielli A, Friedman W.editorsTextbook of neurointensive care. Philadelphia: Saunders; 2004. p.
28. Lee K.editorsThe neuroICU book. New York: McGraw-Hill Medical; 2012. p.
29. Lee VH, Oh JK, Mulvagh SL, Wijdicks EF. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006. 5: 243-9
30. Lindekleiv H, Sandvei MS, Romundstad PR, Wilsgaard T, Njølstad I, Ingebrigtsen T. Joint effect of modifiable risk factors on the risk of aneurysmal subarachnoid hemorrhage: A cohort study. Stroke. 2012. 43: 1885-9
31. Lipsman N, Tolentino J, Macdonald RL. Effect of country or continent of treatment on outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2009. 111: 67-74
32. Lo B, Fukuda H, Nishimura Y, Wan Y, Lo A.editorsBrain-body interactions: Contemporary outcome prediction in aneurysmal subarachnoid hemorrhage using bayesian neural networks and fuzzy logic. New York: Nova Biomedical; 2014. p.
33. Lo B, Macdonald R, Baker A, Levine M. Clinical outcome prediction in aneurysmal subarachnoid hemorrhage using Bayesian neural networks with fuzzy logic inferences. Comput Math Methods Med 2013. 2013. p.
34. Mangieri P, Suzuki K, Ferreira M, Domingues L, Casulari LA. Evaluation of pituitary and thyroid hormones in patients with subarachnoid hemorrhage due to ruptured intracranial aneurysm. Arq Neuropsiquiatr. 2003. 61: 14-
35. Mazarati A. Cytokines: A link between fever and seizures. Epilepsy Curr. 2005. 5: 169-70
36. Mrozek S, Vardon F, Geeraerts T. Brain temperature: Physiology and pathophysiology after brain injury. Anesthesiol Res Pract 2012. 2012. p.
37. O’Carroll RE, Hayes PC, Ebmeier KP, Dougall N, Murray C, Best JJ. Regional cerebral blood flow and cognitive function in patients with chronic liver disease. Lancet. 1991. 337: 1250-3
38. Park J, Woo H, Kang D, Kim YS, Kim MY, Shin IH. Formal protocol for emergency treatment of ruptured intracranial aneurysms to reduce in-hospital rebleeding and improve clinical outcomes. J Neurosurg. 2015. 122: 383-91
39. Payen J, Fauvage B, Falcon D, Lavagne P. Brain oedema following blood-brain barrier disruption: Mechanisms and diagnosis. Ann Fr Anesth Reanim. 2003. 22: 220-5
40. Rowland L, Pedley T, Merritt H.editorsMerritt's neurology. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. p.
41. Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br J Anaesth. 2012. 109: 315-29
42. Sadamasa N, Koyanagi M, Fukuda H, Chin M, Handa A, Yamagata S. Is aneurysm repair justified for the patients aged 80 or older after aneurysmal subarachnoid hemorrhage?. J Neurointerv Surg. 2014. 6: 664-
43. Schneider H, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA. 2007. 298: 1429-38
44. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology. 2010. 21: 128-38
45. Todd MM, Hindman BJ, Clarke WR, Torner JC, Weeks JB, Bayman EO. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2009. 64: 897-908
46. Torbey M.editorsNeurocritical care. Cambridge; New York: Cambridge University Press; 2010. p.
47. Varelas P.editorsSeizures in critical care: A guide to diagnosis and therapeutics. Totowa, NJ: Humana Press; 2005. p.
48. Wijdicks E.editorsThe practice of emergency and critical care neurology. New Work: Oxford University Press; 2010. p.
49. Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010. 2: CD006778-