- Department of Neurosurgery, GB Pant Institute of Post Graduate Medical Education and Research, New Delhi, India.
- Department of Biochemistry, GB Pant Institute of Post Graduate Medical Education and Research, New Delhi, India.
Correspondence Address:
Anil Kumar B. C., Department of Neurosurgery, GB Pant Institute of Post Graduate Medical Education and Research, New Delhi, India.
DOI:10.25259/SNI_889_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nidhi Yadav1, Keshav Mishra1, Anil Kumar B. C.1, Daljit Singh1, Manju Subberwal2. Clinical utility of serum glial fibrillary acidic protein in glial neoplasm. 30-Dec-2022;13:601
How to cite this URL: Nidhi Yadav1, Keshav Mishra1, Anil Kumar B. C.1, Daljit Singh1, Manju Subberwal2. Clinical utility of serum glial fibrillary acidic protein in glial neoplasm. 30-Dec-2022;13:601. Available from: https://surgicalneurologyint.com/surgicalint-articles/12082/
Abstract
Background: Glial fibrillary acidic protein (GFAP) is a member of the cytoskeletal protein family and is widely expressed in astroglial and neural stem cells, also in glial tumors such as astrocytoma and Glioblastoma (GBM). Increased GFAP expression and disruption of the blood–brain barrier are the characteristic features of GBM. Higher serum GFAP levels can help differentiate GBM from GBM mimics (such as primary central nervous system lymphoma, metastasis, or demyelinating lesions).
Methods: This prospective study was carried out in a tertiary care center in the department of neurosurgery on newly diagnosed glioma patients who underwent surgery from January 2018 to July 2019, excluded patients with history of the previous surgery for glioma, traumatic brain injury, and ischemic or hemorrhagic stroke. The blood sample was obtained at admission before undergoing invasive procedure. Pathological examination of the tumor biopsy sample was carried out using classical hematoxylin-eosin and immunohistochemical staining. All statistical analyses were performed using SPSS version 24.0.
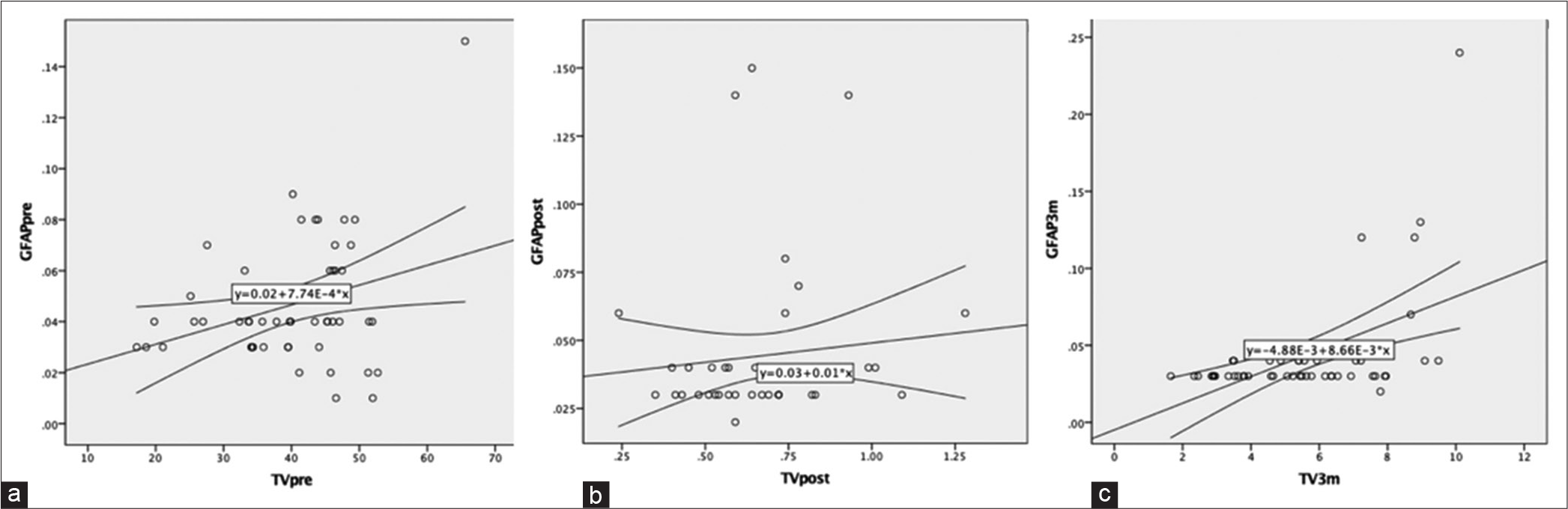
Results: The mean preoperative tumor volume was 40 cm3 (range 17.19–65.57 cm3; standard deviation [SD] = 9.99 cm3) which showed 98.25% mean reduction in volume postsurgery (mean tumor volume = 0.7 cm3; SD = 0.19 cm3). Preoperative serum GFAP measurements show higher levels (spearman’s rho coefficient = 0.610 with P = 0.000) with increasing grade of tumor. GFAP levels also demonstrated higher value with increasing preoperative tumor volume.
Conclusion: Increasing serum GFAP levels in the preoperative period correlate with higher tumor grade, especially grade III and grade IV tumors. The serum GFAP levels showed relation to tumor volume, both before and after surgery.
Keywords: Central Nervous System( CNS), Glial fibrillary acidic protein (GFAP), Glioblastoma (GBM), Magnetic Resonance Imaging ( MRI)
INTRODUCTION
Eng et al. first described glial fibrillary acidic protein (GFAP), a member of the cytoskeletal protein family and have widely expressed in astroglial and neural stem cells, also expressed in glial tumors such as astrocytoma and glioblastoma (GBM).[
GFAP is highly specific for cells with astrocytic differentiation and is widely used as a reliable marker in the immunohistochemical diagnosis and differentiation of brain tumors. Serum GFAP levels are also elevated in cases of brain trauma or hemorrhagic stroke.[
With the majority of studies concentrating on correlation between GBM and serum GFAP level, GFAP also has a potential role in largely unexplored areas such as monitoring therapy, detecting recurrence, and predicting the prognosis.[
MATERIALS AND METHODS
This prospective study was carried out in a tertiary care center in the department of neurosurgery on newly diagnosed glioma patients who underwent surgery from January 2018 to July 2019 after approval of the Institutional Ethical Committee. This study excluded patients with a history of the previous surgery for glioma, prior traumatic brain injury, and concomitant ischemic or hemorrhagic stroke.
The first blood sample was obtained at admission before undergoing any invasive diagnostic or therapeutic procedure. The second blood sample was obtained on the 7th postoperative day and the third at 3 months after surgery. Serum samples were centrifuged immediately in the laboratory, and supernatants were stored at −70°C for serum GFAP level measurement. Serum GFAP level was determined using a biotin-labeled antibody-based sandwich enzyme immunoassay for the quantitative measurement of GFAP. The total assay time was about 5 h and results were expressed as ng/mL.
Presurgical MR imaging of brain including standard sequences (T1-weighted before and after contrast, T2-weighted and fluid-attenuated inversion recovery sequence) was performed. The total tumor volume was estimated from preoperative MRI brain images using the modified ellipsoid formula (A × B × C)/2 with A, B, and C representing the maximum dimensions of the tumor or the necrotic area within the three-perpendicular axis. Residual tumor volume was evaluated using an MRI done within 48 h of surgery. Clinical evaluation and MRI brain were again repeated 3 months after surgery.
Pathological examination of the tumor biopsy sample was carried out using classical hematoxylin-eosin and immunohistochemical staining (including GFAP). Immunohistochemical staining of tumors for GFAP expression was ranked as <25%, 25–50%, 51–75%, and >75% GFAP-positive tumor cells.
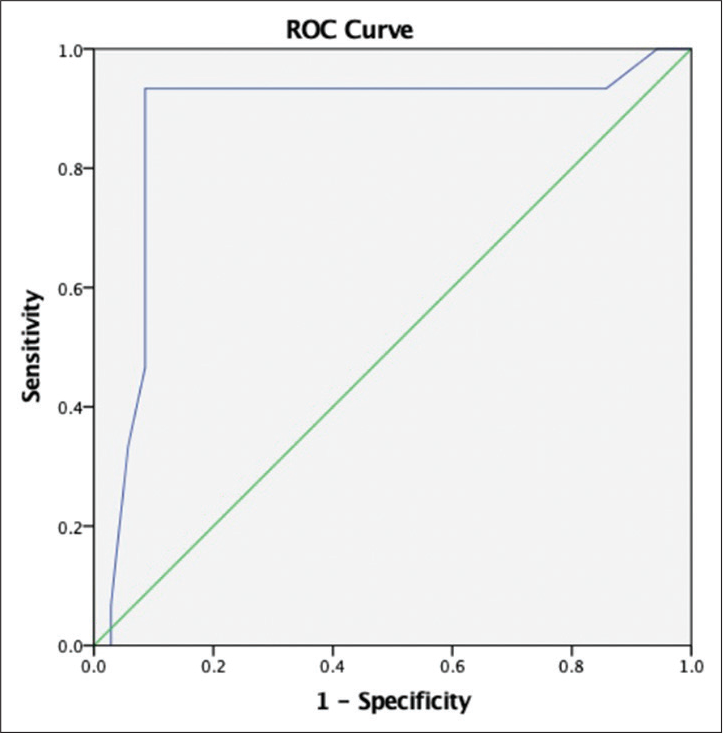
The study data were collected and compiled in Microsoft Excel. All statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, New York, USA). The categorical variables are presented as numbers and percentages, and the continuous variables were described as mean values, medians, and standard deviations (SDs). Paired t-test was used for univariate analysis and correlation was calculated using the Spearman correlation coefficient. ROC curves were used to analyze the cutoff value for serum GFAP.
RESULTS
Fifty patients (mean age: 39.5 years, 12 females) diagnosed with glial tumors were enrolled in this study. Headache (n = 28; 56%) was the most common symptom, followed by seizure (n = 20) and memory loss. The mean time of presentation from symptom onset was 7.1 months. The most common tumor subgroup was WHO grade III (Anaplastic astrocytoma, and anaplastic oligodendroglioma; n = 19) followed by the WHO grade IV tumors (GBM; n = 15) [
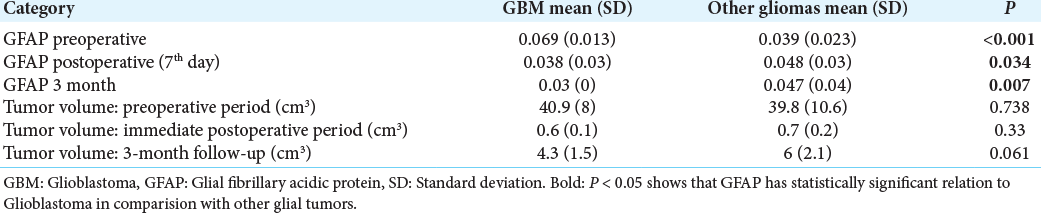
The GBM subgroup presented at older age (GBM: mean age = 54.8 years; non-GBM: mean age 35.2 years vs.; P = 0.000) with seizure (n = 19) was the most common presenting complaint in this group.
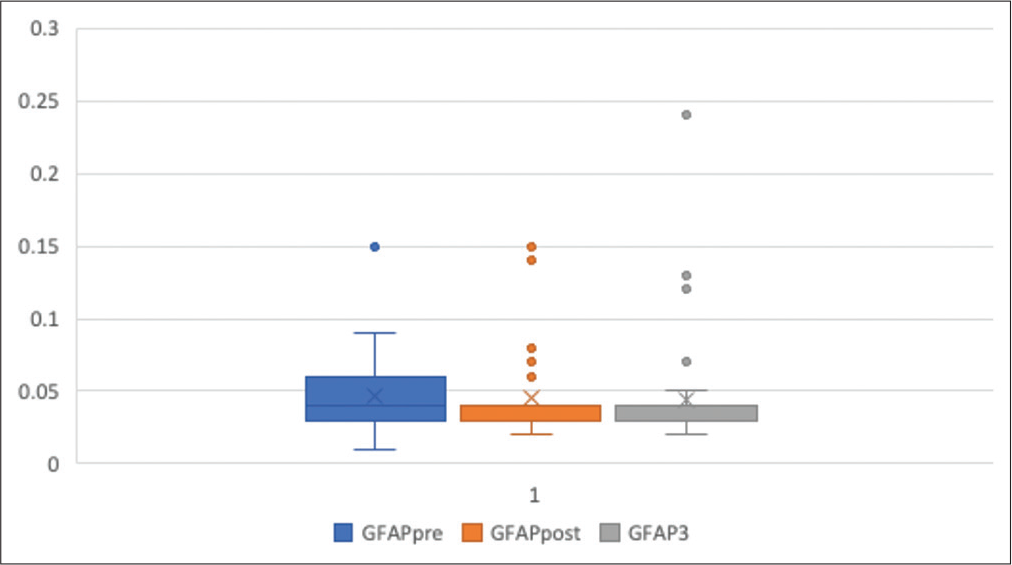
The mean preoperative tumor volume was 40 cm3 (range 17.19–65.57 cm3; SD 9.99 cm3) which showed 98.25% mean reduction in volume postsurgery (mean tumor volume = 0.7 cm3; SD = 0.19 cm3). However, at the end of 3-month follow-up period, mean tumor volume increased to 5.6 cm3 (SD = 2.07 cm3, range = 1.06–9.47 cm3). The increase in volume was more significant in the GBM (mean = 0.06, CI = 0.06–0.07) group compared to the nonGBM group (mean = 0.04 cm3, CI = 0.03–0.05) (P = 0.013). The immunohistochemical staining of tumor using GFAP showed 25–50% immunostaining in 50% of the cases with 28% showing <25% staining. The mean preoperative serum GFAP level was 0.046 ng/mL (range = 0.01–0.15; SD = 0.024) which remained largely unchanged in the immediate postoperative period (mean = 0.044 ng/mL; range = 0.02–0.15; SD = 0.027; P = 0.691) and at 3-month interval (mean = 0.043 ng/mL; range = 0.02–0.24; SD = 0.036; P = 0.809) [
Preoperative serum GFAP measurements show higher levels (spearman’s rho coefficient = 0.610 with P = 0.000) with increasing grade of tumor, as shown in
Figure 3:
(a) Scatter plot showing relation of preoperative serum glial fibrillary acidic protein (GFAP) levels with preoperatively tumor volume, (b) scatter plot showing relation of postoperative serum GFAP levels with immediate postoperative tumor volume, and (c) scatter plot showing relation of serum GFAP levels and tumor volume at 3-month postoperative follow-up.
On subgroup analysis, immediate postoperative GFAP levels (0.038 ng/mL) showed a 44.92% decrease from the preoperative level of 0.069 ng/mL which, further, reduced at 3-month interval (0.03 ng/mL; P = 0.028). In the non-GBM group, the serum GFAP level demonstrated an unexpected increase from 0.039 ng/mL to 0.048 ng/mL in the immediate postoperative period [
DISCUSSION
This study found that serum GFAP levels are elevated in patients with a diagnosis of GBM and these levels are demonstrably higher than that in grade II or grade III tumors (GFAP-positive cases: GBM-93.3% vs. non-GBM-8.5%; P = 0.00). In the previous studies, it is suggested that increased astroglial turnover and damage to blood–brain barrier are the dominant mechanisms responsible for elevated GFAP serum levels in GBM population compared to increased GFAP expression by tumor cells. This is, further, substantiated by the fact that cellular GFAP expression does not significantly differ between normal brain and GBM samples.[
Serial variations in serum GFAP level
Overall serum GFAP levels did not show a marked variation between preoperative to immediate postoperative period (0.046–0.044 ng/mL; P = 0.691) to 3-month interval (0.044–0.043 ng/mL; P = 0.809). However, on subgroup analysis, the GBM group showed a significant drop in serum GFAP levels postoperatively (0.069–0.038 ng/mL; P = 0.000) which demonstrated further decrease at 3-month follow-up (0.038–0.030 ng/mL; P = 0.028). Vietheer and colleagues found reduced serum GFAP levels compared to baseline at 6 weeks postsurgery which is similar to our observationsl.[
Correlation of GFAP with tumor volume
A positive correlation was observed between the tumor volume and GFAP level in the immediate postoperative period (spearman’s rho coefficient = 0.306; P = 0.031) as well as at a 3-month interval (spearman’s rho coefficient = 0.384; P = 0.006). Other authors have also found a positive correlation between serum GFAP levels with tumor volume.[
Variation of GFAP with grade
Our study revealed preoperative serum GFAP levels to correlate with tumor grade which is similar to the findings of Jung et al. who detected higher levels of serum GFAP levels in grade IV astrocytoma compared to other glial tumors.[
GFAP in differentiating glioma from non-glial tumors
Significantly elevated serum GFAP levels were found in grade IV astrocytoma compared to grade II and III tumors, intracranial metastasis, as well as healthy controls.[
CONCLUSION
Increasing serum GFAP levels in the preoperative period correlate with a higher tumor grade, especially grade III and grade IV tumors. The serum GFAP levels also showed a relation to tumor volume, both before and after surgery. A cutoff level of more than 0.05 ng/mL can be used to distinguish GBM from other glial tumors. However, serum GFAP levels could not predict residual tumor, nor did it show any relation to tumor progression, but further study with larger sample size is warranted to explore the role of any potential threshold phenomenon. This serum marker helps for the detection of glioma, particularly when there is a diagnostic dilemma on radiology. Moreover, the serum biomarker would save the patient from unneccessory radiation exposure in the follow-up to detect any recurrence and can differentiate radionecrosis from recurrence. The serum GFAP level will not be the replacement for the histopathology at confirmation of glioma in the beginning, however in recurrent glioma and for differentiation of radionecrosis, it would be of immense value. Once in routine practice, it will be much more cost effective than repeated radiology (CT/MRI scan), saving the patient from hassle of taking the patient to CT/MRI center, it will be of phenomenal comfort for bed ridden patient.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bazarian JJ, Biberthaler P, Welch RD, Lewis LM, Barzo P, Bogner-Flatz V. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): A multicentre observational study. Lancet Neurol. 2018. 17: 782-9
2. Diagnostic Accuracy of Plasma Glial Fibrillary Acidic Protein for Differentiating Intracerebral Hemorrhage and Cerebral Ischemia in Patients with Symptoms of Acute Stroke Clinical Chemistry Oxford Academic. Available from: https://www.academic.oup.com/clinchem/article/58/1/237/5620623?login=false [Last accessed on 2022 Sep 18].
3. Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM. Acute biomarkers of traumatic brain injury: Relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014. 31: 19-25
4. Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res. 2000. 25: 1439-51
5. Gállego Pérez-Larraya J, Paris S, Idbaih A, Dehais C, Laigle-Donadey F, Navarro S. Diagnostic and prognostic value of preoperative combined GFAP, IGFBP-2, and YKL-40 plasma levels in patients with glioblastoma. Cancer. 2014. 120: 3972-80
6. Gandhoke CS, Shah AS, Singh D, Subberwal M, Gupta RK, Gupta VK. Whether serum glial fibrillary acidic protein (GFAP) can be used as a diagnostic biomarker in patients with Glioblastoma?. MAMC J Med Sci. 2020. 6: 27-32
7. Ilhan-Mutlu A, Wagner L, Widhalm G, Wöhrer A, Bartsch S, Czech T. Exploratory investigation of eight circulating plasma markers in brain tumor patients. Neurosurg Rev. 2013. 36: 45-55 discussion 55-6
8. Jung CS, Foerch C, Schänzer A, Heck A, Plate KH, Seifert V. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007. 130: 3336-41
9. Kiviniemi A, Gardberg M, Frantzén J, Parkkola R, Vuorinen V, Pesola M. Serum levels of GFAP and EGFR in primary and recurrent high-grade gliomas: Correlation to tumor volume, molecular markers, and progression-free survival. J Neurooncol. 2015. 124: 237-45
10. Pre-and Early Postoperative GFAP Serum Levels in Glioma and Brain Metastases. Available from: https://www.pubmed.ncbi.nlm.nih.gov/29797180 [Last accessed on 2022 Sep 18].
11. Thelin EP, Zeiler FA, Ercole A, Mondello S, Büki A, Bellander BM. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: A systematic review. Front Neurol. 2017. 8: 300
12. Tichy J, Spechtmeyer S, Mittelbronn M, Hattingen E, Rieger J, Senft C. Prospective evaluation of serum glial fibrillary acidic protein (GFAP) as a diagnostic marker for glioblastoma. J Neurooncol. 2016. 126: 361-9
13. Vietheer JM, Rieger J, Wagner M, Senft C, Tichy J, Foerch C. Serum concentrations of glial fibrillary acidic protein (GFAP) do not indicate tumor recurrence in patients with glioblastoma. J Neurooncol. 2017. 135: 193-9