- Department of Histopathology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Radiation Oncology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Internal Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Bishan Dass Radotra
Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_314_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bishan Dass Radotra1, Mayur Parkhi1, Debajyoti Chatterjee1, Budhi Singh Yadav2, Nagarjun Rao Ballari2, Gaurav Prakash3, Sunil Kumar Gupta4. Clinicopathological features of primary central nervous system diffuse large B cell lymphoma: Experience from a Tertiary Center in North India. 11-Dec-2020;11:424
How to cite this URL: Bishan Dass Radotra1, Mayur Parkhi1, Debajyoti Chatterjee1, Budhi Singh Yadav2, Nagarjun Rao Ballari2, Gaurav Prakash3, Sunil Kumar Gupta4. Clinicopathological features of primary central nervous system diffuse large B cell lymphoma: Experience from a Tertiary Center in North India. 11-Dec-2020;11:424. Available from: https://surgicalneurologyint.com/surgicalint-articles/10452/
Abstract
Background: Primary central nervous system-diffuse large B-cell lymphoma (PCNS-DLBCL) is a rare extra-nodal Non-Hodgkin lymphoma. There is relative paucity of literature on PCNSL from Indian subcontinent. We aimed to analyze the clinicopathological features of PCNSL and categorize them into germinal center B cell (GCB) and non-GCB subtypes to assess their prognostic significance in Indian context.
Methods: All patients with histopathologically diagnosed PCNSLs at our center over a period of 6 years were recruited and classified into GCB and non-GCB using Han’s algorithm (immunohistochemistry for CD10, BCL6 and MUM1). In situ hybridization (ISH) for Epstein-Barr virus (EBV)-encoded RNA was performed.
Results: Eighty-six cases of PCNS-DLBCL were included with median age of 55 years. Majority of them were supratentorial in location (n = 62). All patients were immunocompetent. On immunohistochemical assessment, 69 (80.2%) were of NGCB subtype, 10 (11.6%) were of GCB subtype, and 7 (8.1%) were unclassified. Overall, MUM1, BCL-6, and CD10 expressions were seen in 69 (80.2%), 28 (32.6%), and 2 cases (2.3%), respectively. Four cases (4.6%) showed C-MYC expression. The median overall survival (OS) was 675 days. None of the factors (age, sex, location, immunomarkers, and GCB vs. NGCB phenotype) showed correlation with OS; however, BCL6 positive cases showed slight better OS (P > 0.05). All cases were negative for EBV-LMP1 on ISH.
Conclusion: The majority of the CNS DLBCL belongs to non-GCB phenotype and uniformly carry poor prognosis, irrespective of their phenotype. Individual markers, such as BCL-6, MUM1, or CD10, are unable to predict outcome in PCNS-DLBCL.
Keywords: Central nervous system, Diffuse large B-cell lymphoma, Han’s algorithm, Immunocompetent, Non-germinal center B cell type
INTRODUCTION
Primary central nervous system (PCNS) lymphomas (PCNSLs) are extranodal, malignant non-Hodgkin lymphomas that are confined to the brain, eyes, leptomeninges, or spinal cord, in the absence of systemic lymphoma.[
Systemic nodal DLBCL is divided into two subgroups named germinal center B-cell (GCB) such as and non-GCB such as using cDNA microarray and immunomarkers (CD10, BCL6, and MUM1), according to Hans et al. algorithm.[
MATERIALS AND METHODS
Study population, samples, and clinical data
The surgical pathology database of single institution (Post Graduate Institute of Medical Education and Research, Chandigarh, India) was searched for cases of PCNS-DLBCL, from January1, 2014, to December 31, 2019 (6 years). Cases of systemic DLBCL with secondary CNS involvement were excluded.[
Tissue microarrays (TMA) and immunohistochemistry (IHC)
TMA were constructed by punching cores of 2 mm from formalin fixed paraffin embedded blocks of cases with adequate tissue sample and were inserted in a grid pattern into a recipient paraffin block using an automated tissue microarrayer (Quick Ray Master UATM-272B, Korea). TMA blocks contained three cores from each case. Tonsillar tissue was included as internal control. A hematoxylin and eosin stained slide of each TMA block was generated to assess their quality. For cases where TMA could be prepared, IHC was performed on TMA blocks. Cases with limited material such as stereotactic biopsy and brain biopsy with small amount of tumor were not included in TMA, but immunohistochemical study was performed on whole slides. IHC was carried out on TMA block for 46 cases and on whole slide in 40 cases. For IHC, 5-μm sections of a representative block were obtained. The following antibodies were used: glial fibrillary acidic protein (GFAP; clone EP672Y, Cell Marque, dilution 1:100), leukocyte common antigen (LCA; clone 2B11+PD7/26, Dako, dilution 1:100), CD3 (Rabbit polyclonal, Cell Marque, dilution 1:500), CD20 (clone L26, Dako, dilution 1:300), CD10 (clone 56C6, Cell Marque, dilution 1:20), B-cell lymphoma 6 (BCL6; cone G1191E/A8, Cell Marque, dilution 1:300), Multiple Myeloma 1 (MUM1; clone MRQ-43, Cell Marque, dilution 1:300), B-cell lymphoma 2 (BCL2; clone 124, Dako, dilution 1:50), c-myc (clone EP121, Cell Marque, dilution 1:50), and Ki-67 (clone SP6, Cell Marque, dilution 1:300). These were performed on Ventana, Biotek automated system with appropriate positive and negative controls run concurrently. Briefly, paraffin sections were mounted on charged glass slides, air-dried over-night, and then deparaffinized. To enhance the immunostaining, a heat-induced epitope-retrieval procedure was performed. After incubation with blocking serum, sections were incubated with primary antibodies, followed by a biotinylated polyvalent secondary antibody solution. Sections were then incubated with horseradish peroxidase conjugated avidinbiotin complex, followed by 3,3-diaminobenzidine and hydrogen peroxidase.
Three pathologists independently evaluated the stained slides. For each immunostain, the percentage of positive cells was estimated for each of the 10 high power fields evaluated, and an average calculated. Immunostaining for CD10, BCL-6, and MUM1 was considered positive if >30% of the tumor cells were immunoreactive. The intensity of staining was also evaluated but was not used to determine positivity because the variability in tissue fixation and processing appeared to affect the intensity of staining. Sub-classification was carried out as described earlier, according to the schema proposed by Hans et al.[
In situ hybridization (ISH) for Epstein-Barr virus (EBV)
ISH for EBV-encoded RNA (EBER-1) (Ventana) was performed on formalin-fixed paraffin-embedded tissue sections for all cases with sufficient tissue with the appropriate positive and negative controls. All cases were assessed for EBV-encoded LMP1 by IHC.
Statistical analysis
The results were analyzed by employing appropriate statistical methods. Group comparisons were performed with Fisher’s exact test. Overall survival (OS) was defined as the time from diagnosis to death from any cause. OS was estimated using the Kaplan–Meier method, and group comparisons were made with the log-rank test.
RESULTS
Of total 101 cases of PCNS-DLBCL obtained from the archives, 15 were excluded due to inadequate material (n = 9) and non-availability of blocks (n = 6). Thus, a total 86 cases were included for final analysis.
Clinical findings
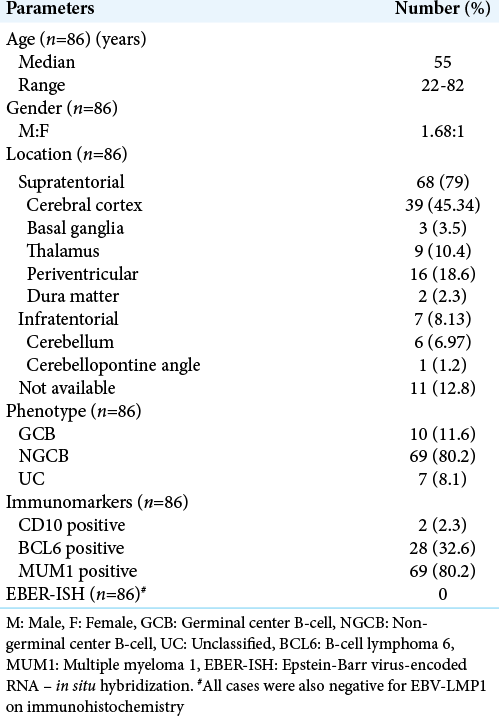
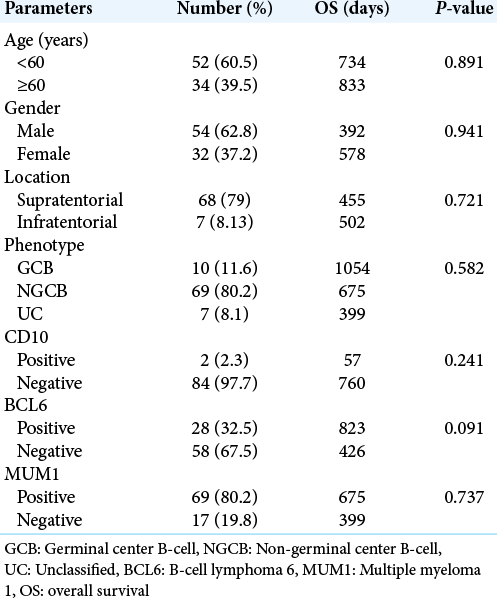
Out of 86 cases, 56 were male (62.8%) and 32 were female (37.2%) patients with M:F ratio of 1.68:1. The median age was 55 years (range: 22–82 years) with 34 patients of elderly (≥60 years) age group. Majority of these cases were supratentorial (n = 68) in location while only seven cases were infratentorial. Site could not be determined in 11 cases as it was not mentioned in the biopsy request form. All patients tested negative for HIV by enzyme immunoassay. None of the patients had history of organ transplantation, chronic illness, or any other form of immunodeficiency. Five patients had other coexisting diseases, which included chronic hepatitis due to Hepatitis B virus (n = 3), cytomegalovirus colitis (n = 1), and Type 2 diabetes mellitus (n = 1). Follow-up was performed for 66 patients, as 20 were lost to follow-up.
Histology and IHC
Our material consisted of both stereotaxic (n = 35, 40.7%), and open (n = 51, 59.3%) biopsies. In all cases, the brain parenchyma was replaced and diffusely infiltrated by tumor in prominent sheet-like pattern. The histology revealed characteristic angiocentric distribution, large pleomorphic nuclei with irregular thick nuclear membrane, prominent nucleoli, brisk mitotic figures, and apoptotic bodies. Most of the cases showed areas of geographic necrosis. Five cases showed interspersed histiocytes, giving starry sky appearance. No case showed plasmacytoid morphology. The tumor cells showed immunoreactivity for CD20 (cell membrane; diffuse and intense) but were completely negative for CD3 and GFAP immunostains.
On IHC assessment, 69 (80.2%) were of the NGCB subtype, 10 (11.6%) were of germinal center (GCB) subtype, and 7 (8.1%) were unclassified (UC). The median age for NGCB, GCB and UC was 55 years, 51.5 years, and 53 years, respectively (P = 0.98). Among NGCB subtype, all the cases (n = 69) showed nuclear positivity for MUM1. Eighteen of these MUM1 positive cases also demonstrated nuclear immunoreactivity for BCL6; one of which displayed BCL2 cytoplasmic positivity. Four MUM1 positive cases expressed c-MYC and none of them showed BCL6 immunoreactivity. Ten cases (11.6%) were categorized as GCB type, and all showed nuclear BCL6 expression and were negative for MUM1. Two of the ten GCB cases were CD10 positive (membranous). Seven cases could not be classified using Han’s algorithm. They did not show expression of either MUM-1, BCL-6, or CD10. The Ki-67 labeling index ranged between 70 and 100% with median of 87.5% [
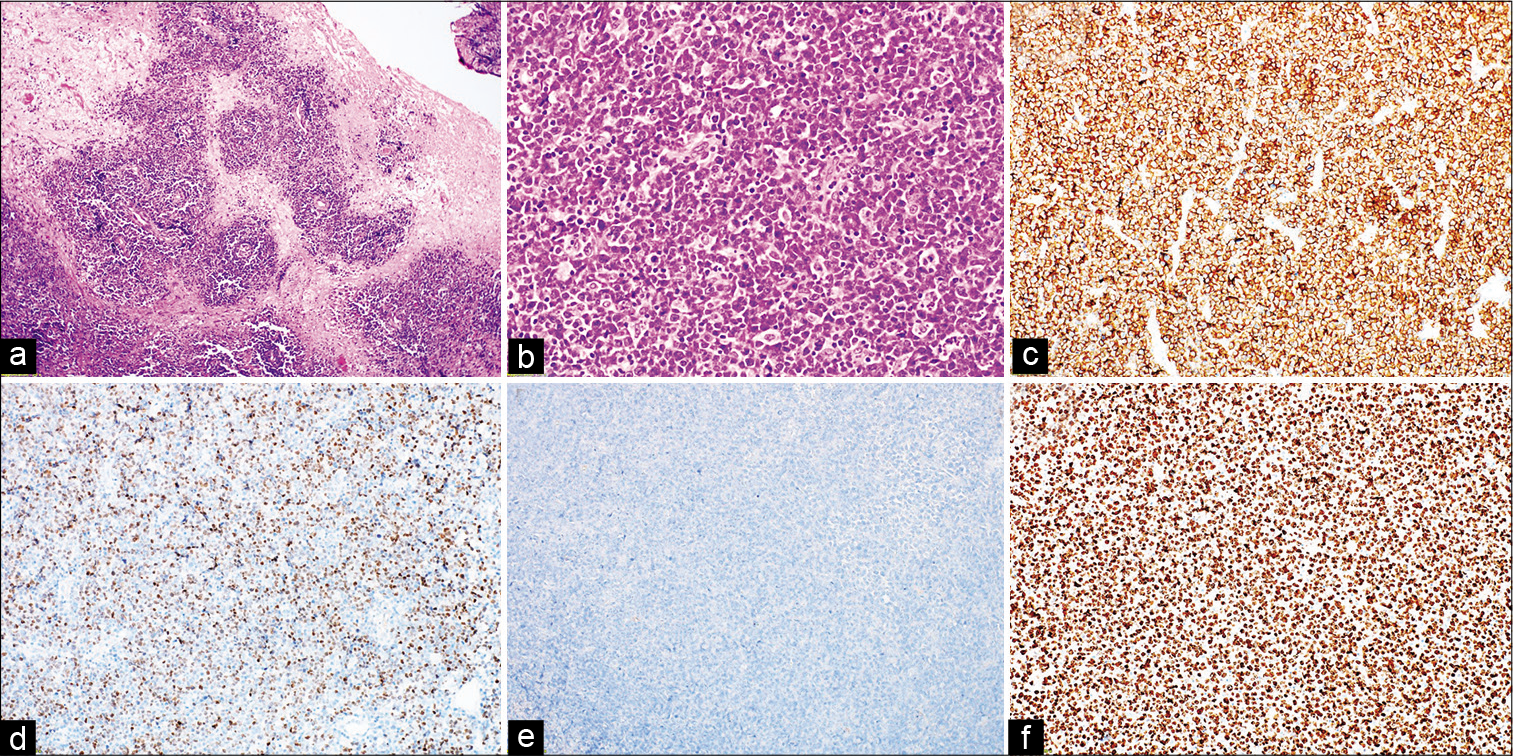
Figure 1:
Primary diffuse large B-cell lymphoma of central nervous system, germinal center B-cell phenotype. (a) Characteristic angiocentric distribution is seen (hematoxylin and eosin [H&E], 100×). (b) Tumor cells are arranged in diffuse sheet with starry sky appearance (H&E, 200×). (c) CD20 shows diffuse membranous positivity (immunoperoxidase, 200×). (d) Nuclear expression for BCL6 immunomarker (immunoperoxidase, 200×). (e) MUM1 immunomarker is negative (immunoperoxidase, 200×). (f) Mitotic activity is brisk; Ki-67 proliferation index is more than 90% (immunoperoxidase, 200×).
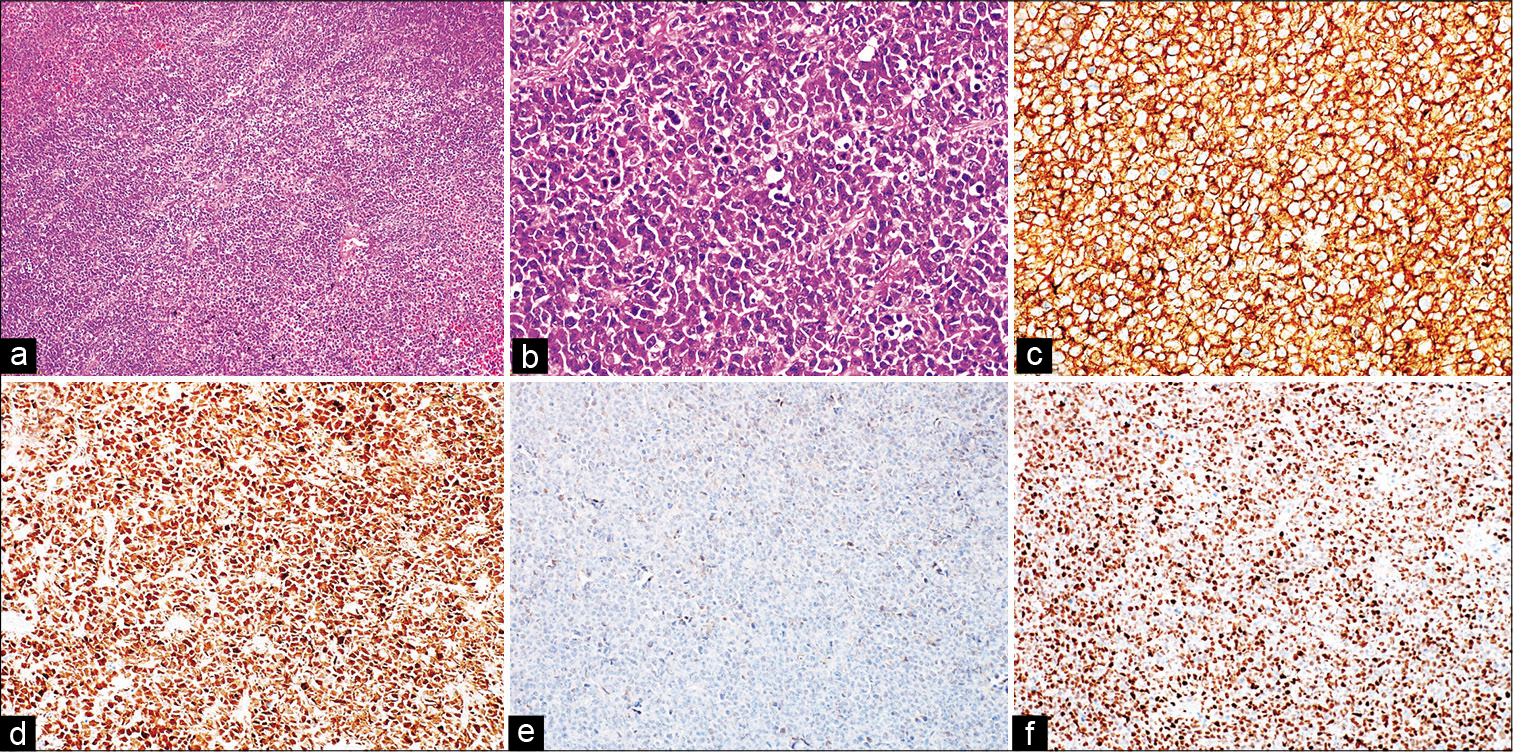
Figure 2:
Primary diffuse large B-cell lymphoma of central nervous system, non-germinal center B-cell phenotype. (a) Tumor cells are arranged in diffuse sheet (hematoxylin and eosin [H&E], 100×). (b) Large B-cell morphology with thick nuclear membrane and single to multiple prominent nucleoli (H&E, 400×). (c) CD20 shows diffuse membranous positivity (immunoperoxidase, 200×). (d) Strong nuclear expression of MUM1 immunostain (immunoperoxidase, 200×). (e) BCL6 immunomarker is negative (immunoperoxidase, 200×). (f) Nuclear expression for c-MYC (immunoperoxidase, 200×).
Treatment and follow-up
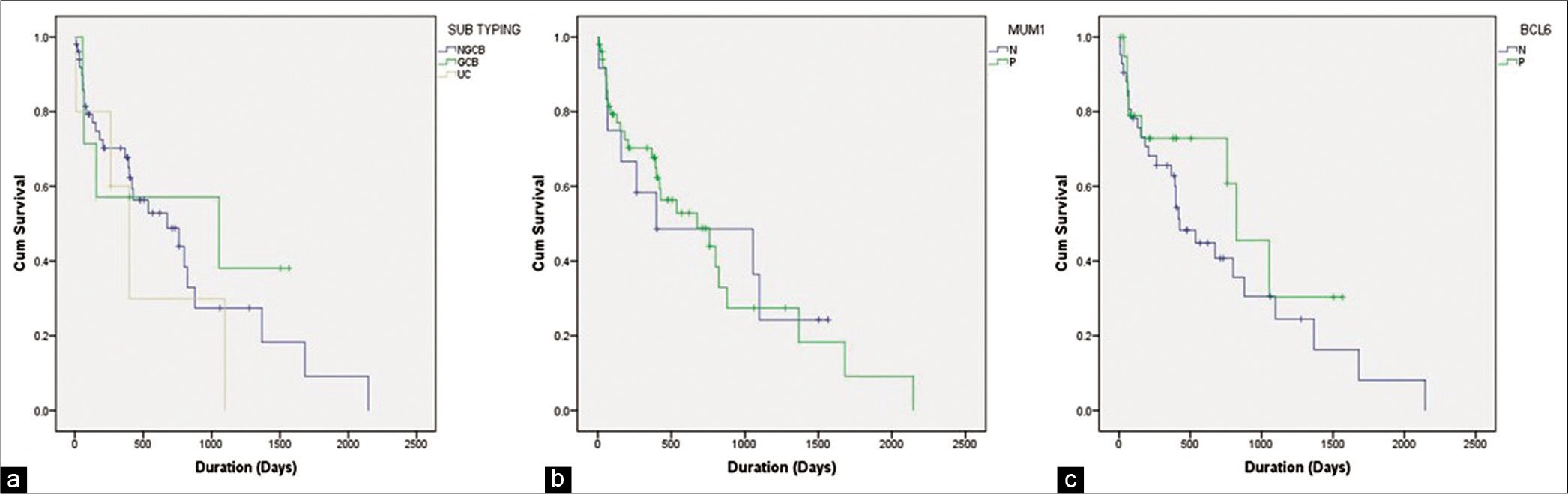
The follow-up data were available in 66 patients (76.74%) with intervals ranging from 322 to 1028 days. The median follow- up time was 675 days. Rest 20 patients were lost to follow-up. The treatment details were available in 66 patients (76.74%) and were in the form of variable combination of radiotherapy and chemotherapy (methotrexate, rituximab, vincristine, and dexamethasone) in 41 patients (62.12%), chemotherapy alone in 13 patients (19.7%), and radiotherapy alone in ten patients (11.62%). Two patients did not receive any therapy because of poor general condition. Thirty of 66 patients (45.45%) were found to be alive (26 NGCB, 3GCB, and 1UC) while 36 of 66 patients (54.54%) were found to be dead (28 NGCB, 4 GCB, and 4UC) at the time of last follow-up. The median OS was 675 days. There was no statistically significant difference in survival between non-germinal center, germinal center, and UC subtypes (Log-Rank chi-square = 1.083, df = 2, P = 0.582). The median OS for MUM1 positive and MUM1 negative cases were 675 and 399 days, respectively (P = 0.966). The median OS for BCL-6 positive and BCL-6 negative cases were 823 and 426 days, respectively (P = 0.301) [
Figure 3:
Kaplan–Meier curve. (a) No statistically significant difference in survival between the germinal center B-cell (GCB), non-GCB and unclassified subgroups. (b) No statistically significant difference in survival between the BCL6 positive and BCL6 negative cases. The patients with BCL6 expression exhibited slight better overall survival. (c) No statistically significant difference in survival between the MUM1 positive and MUM1 negative cases.
DISCUSSION
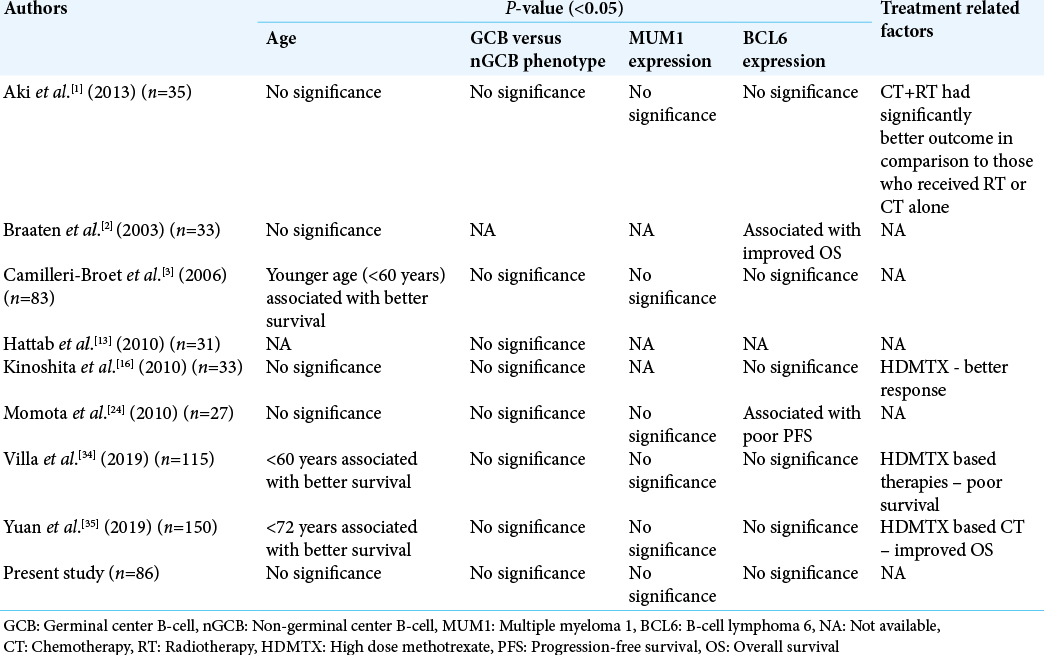
In this study, we analyzed 86 cases of primary CNS DLBCL in tertiary health care center of North India, one of the largest cohorts in Indian population. All the patients were immunocompetent. The median age of presentation was 55 years. Using Han’s algorithm, these cases were further subcategorized into GCB and non-GCB subgroups.[
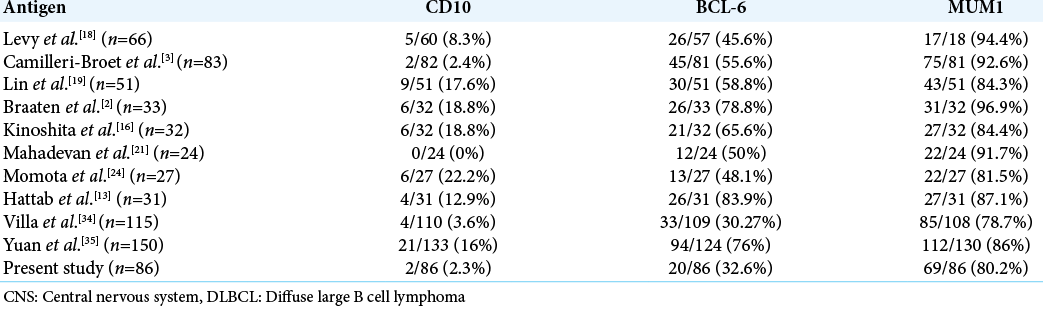
In the present study, most of the cases (80.2%) belonged to non-GCB category which is in agreement with most of the previous studies. Almost all studies have reported a high frequency of MUM1 expression, low frequency of CD10 expression, while the reported frequency of Bcl-6 expression is highly variable [
There is a paucity of literature on PCNSL from Indian subcontinent. In a previous study from our institute, Powari et al.[
In the current study, c-MYC expression was detected in four cases, all of which were MUM1 positive. BCL2 and BCL-6 immunoreactivity was not seen in association with c-MYC expression. Double or triple hit is very uncommon in primary CNS DLBCL. Nosrati et al.[
The prognosis of CNS DLBCL is poor. Compared with systemic DLBCL, primary CNS DLBCL carries a much worse prognosis. Various studies have evaluated different clinical and pathological parameters to predict the prognosis in CNS DLBCL [
This study, as well the previous studies have shown that the biology and behavior of primary CNS DLBCL differ from systemic DLBCL. Compared to systemic DLBCL, most of the CNS DLBCL belong to non-GCB phenotype, and carry uniformly poor prognosis. Even cases with GCB phenotype also carry poor prognosis. Compared to systemic DLBCL, PCNS DLBCL carries an extremely high load of somatic mutations of immunoglobulin genes and other protooncogenes. Based on these findings, some researchers have proposed that PCNS DLBCL is a separate entity. However, comparative genomic hybridization analysis of chromosomal imbalances showed similar abnormalities in PCNSL and systemic DLBCL, arguing against this hypothesis.[
CONCLUSION
To summarize, the majority of the CNS DLBCL belong to non-GCB phenotype and uniformly carries poor prognosis, irrespective of their phenotype. Double or triple hit lymphomas are very rare. Individual markers such as BCL-6, MUM1, or CD10 are unable to predict outcome in PCNS DLBCL. The reason for poor prognosis needs to be further evaluated.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aki H, Uzunaslan D, Saygin C, Batur S, Tuzuner N, Kafadar A. Primary central nervous system lymphoma in immunocompetent individuals: A single center experience. Int J Clin Exp Pathol. 2013. 6: 1068-75
2. Braaten KM, Betensky RA, de Leval L, Okada Y, Hochberg FH, Louis DN. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003. 9: 1063-9
3. Camilleri-Broet S, Criniere E, Broet P, Delwail V, Mokhtari K, Moreau A. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood. 2006. 107: 190-6
4. Chamberlain MC. Long survival in patients with acquired immune deficiency syndrome-related primary central nervous system lymphoma. Cancer. 1994. 73: 1728-30
5. Chang CC, Kampalath B, Schultz C, Bunyi-Teopengco E, Logan B, Eshoa C. Expression of p53, c-Myc, or Bcl-6 suggests a poor prognosis in primary central nervous system diffuse large B-cell lymphoma among immunocompetent individuals. Arch Pathol Lab Med. 2003. 127: 208-12
6. Chang CC, McClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B. Immunohistochemical expression patterns of germinal centre and activation B-cell markers correlate with prognosis in diffuse large B-cell lymphoma. Am J Surg Pathol. 2004. 28: 464-70
7. Cheng J, Tu P, Shi QL, Zhou HB, Zhou ZY, Zhao YC. Primary diffuse large B-cell lymphoma of central nervous system belongs to activated B-cell-like subgroup: A study of 47 cases. Zhonghua Bing Li Xue Za Zhi. 2008. 37: 384-9
8. Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009. 15: 5494-502
9. Corn BW, Marcus SM, Topham A, Hauck W, Curran WJ. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000?. Cancer. 1997. 79: 2409-13
10. Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A. Prognostic scoring system for primary CNS lymphomas: The international extranodal lymphoma study group experience. J Clin Oncol. 2003. 21: 266-72
11. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004. 103: 275-82
12. Harada K, Nishizaki T, Kubota H, Harada K, Suzuki M, Sasaki K. Distinct primary central nervous system lymphoma defined by comparative genomic hybridization and laser scanning cytometry. Cancer Genet Cytogenet. 2001. 125: 147-50
13. Hattab EM, Martin SE, Al-Khatib SM, Kupsky WJ, Vance GH, Stohler RA. Most primary central nervous system diffuse large B-cell lymphomas occurring in immunocompetent individuals belong to the nongerminal center subtype: A retrospective analysis of 31 cases. Mod Pathol. 2010. 23: 235-43
14. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Ruda R. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European association for neuro-oncology. Lancet Oncol. 2015. 16: e322-32
15. Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002. 95: 193-202
16. Kinoshita M, Hashimoto N, Izumoto S, Okita Y, Kagawa N, Maruno M. Immunohistological profiling by B-cell differentiation status of primary central nervous system lymphoma treated by high-dose methotrexate chemotherapy. J Neurooncol. 2010. 99: 95-101
17. Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013. 9: 317-27
18. Levy O, Deangelis LM, Filippa DA, Panageas KS, Abrey LE. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2008. 112: 151-6
19. Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang JH, Chang KC. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006. 12: 1152-6
20. Louis D, Ohgaki H, Wiestler O, Cavenee W.editors. WHO Classification of Tumours of the Central Nervous System WHO/IARC Classification of Tumours, Revised. Geneva: World Health Organization; 2016. p.
21. Mahadevan A, Rao CR, Shanmugham M, Shankar SK. Primary central nervous system diffuse large B-cell lymphoma in the immunocompetent: Immunophenotypic subtypes and Epstein-Barr virus association. J Neurosci Rural Pract. 2015. 6: 8-14
22. Manoj N, Arivazhagan A, Mahadevan A, Bhat DI, Arvinda HR, Devi BI. Central nervous system lymphoma: Patterns of incidence in Indian population and effect of steroids on stereotactic biopsy yield. Neurol India. 2014. 62: 19-25
23. Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011. 29: 200-7
24. Momota H, Narita Y, Maeshima AM, Miyakita Y, Shinomiya A, Maruyama T. Prognostic value of immunohistochemical profile and response to high-dose methotrexate therapy in primary CNS lymphoma. J Neurooncol. 2010. 98: 341-8
25. Nosrati A, Monabati A, Sadeghipour A, Radmanesh F, Safaei A, Movahedinia S. MYC, BCL2, and BCL6 rearrangements in primary central nervous system lymphoma of large B cell type. Ann Hematol. 2019. 98: 169-73
26. Patel B, Chacko G, Nair S, Anandan J, Chacko AG, Rajshekhar V. Clinicopathological correlates of primary central nervous system lymphoma: Experience from a tertiary care center in South India. Neurol India. 2015. 63: 77-82
27. Powari M, Radotra B, Das A, Banerjee AK. A study of primary central nervous system lymphoma in Northern India. Surg Neurol. 2002. 57: 113-6
28. Preusser M, Woehrer A, Koperek O, Rottenfusser A, Dieckmann K, Gatterbauer B. Primary central nervous system lymphoma: A clinicopathological study of 75 cases. Pathology. 2010. 42: 547-52
29. Raoux D, Duband S, Forest F, Trombert B, Chambonniere ML, Dumollard JM. Primary central nervous system lymphoma: Immunohistochemical profile and prognostic significance. Neuropathology. 2010. 30: 232-40
30. Rickert CH, Dockhorn-Dworniczak B, Simon R, Paulus W. Chromosomal imbalances in primary lymphomas of the central nervous system. Am J Pathol. 1999. 155: 1445-51
31. Sarkar C, Sharma MC, Deb P, Singh R, Santosh V, Shankar SK. Primary central nervous system lymphoma-a hospital based study of incidence and clinicopathological features from India (1980-2003). J Neurooncol. 2005. 71: 199-204
32. Sharma MC, Gupta RK, Kaushal S, Suri V, Sarkar C, Singh M. A clinicopathological study of primary central nervous system lymphomas and their association with Epstein-Barr virus. Indian J Med Res. 2016. 143: 605-15
33. Swerdlow SH.editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2017. p.
34. Villa D, Tan KL, Steidl C, Ben-Neriah S, Al Moosawi M, Shenkier TN. Molecular features of a large cohort of primary central nervous system lymphoma using tissue microarray. Blood Adv. 2019. 3: 3953-61
35. Yuan XG, Huang YR, Yu T, Xu Y, Liang Y, Zhang XH. Primary central nervous system lymphoma in China: A single-center retrospective analysis of 167 cases. Ann Hematol. 2020. 99: 93-104