- Department of Neurosurgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, Japan,
- Department of Neurosurgery, Faculty of Medicine, Assiut University, Markaz El-Fath, Assiut Governorate, Egypt.
Correspondence Address:
Farrag Mohammad
Department of Neurosurgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, Japan,
DOI:10.25259/SNI_1_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Farrag Mohammad, Takashi Horiguchi, Katsuhiro Mizutani, Kazunari Yoshida. Clipping versus coiling in unruptured anterior cerebral circulation aneurysms. 21-Mar-2020;11:50
How to cite this URL: Farrag Mohammad, Takashi Horiguchi, Katsuhiro Mizutani, Kazunari Yoshida. Clipping versus coiling in unruptured anterior cerebral circulation aneurysms. 21-Mar-2020;11:50. Available from: https://surgicalneurologyint.com/surgicalint-articles/9919/
Abstract
Background: Unruptured intracranial aneurysms (UIAs) are not uncommon, especially in Japan. Treatment strategy for UIAs has evolved in the past decades in Western countries with the increased use of endovascular treatment as the primary option, but in Japan, clipping still has the upper hand.
Methods: This study retrospectively included 200 patients treated by clipping or coiling for UIAs located in the anterior cerebral circulation. Postoperative angiographic and clinical outcomes were evaluated.
Results: Of 200 UIAs, 147 and 53 were treated by surgery and coiling, respectively. The average follow-up duration was 30.2 ± 18.8 months for clipping and 29.3 ± 17.6 months for coiling. Complete occlusion was greater in the surgery group (78.9%) than the endovascular group (18.8%). Regrowth occurred in 1.4% of the clipping group and 13.2% of the coiling group. Ischemic events were encountered in both groups; asymptomatic ones were higher in the coiling group (24.5%) than in the clipping group (2%), while symptomatic ischemic complications were equal (7.5%) in both groups. The deterioration of modified Rankin scale was detected totally in 13 UIAs (6.5%) with no statistical difference between groups. Postoperative hospital period was longer in clipping (P = 0.01).
Conclusion: Clipping and coiling were both safe and feasible in the treatment of unruptured aneurysms. The clipping was advantageous in durability, while the rate of morbidity was lower, and hospitalization period was shorter in the coiling group. The clipping and coiling should coexist while complementing each other by understanding the advantages and disadvantages of both.
Keywords: Anterior circulation, Clipping, Coiling, Unruptured aneurysm
INTRODUCTION
For patients with unruptured intracranial aneurysms (UIAs), the options of treatment are variable, including microsurgical clipping and endovascular coil embolization.[
The detection of UIA in Japanese has been common and increased because of the countrywide development of medical brain checkup systems using magnetic resonance angiography (MRA) for asymptomatic persons. As a result, a large meta-analysis demonstrated that by comparison with populations from North America and European countries, Japanese people had a 2.8 times increased risk of aneurysm rupture.[
There have been several articles concerning ruptured cerebral aneurysms such as ISAT and BRAT, but, for unruptured aneurysms, only one small randomized controlled trial (RCT) has been reported and the optimization of these two treatments has not been well established.[
MATERIALS AND METHODS
We retrospectively reviewed 200 UIAs treated in our institution from January 2012 to December 2016. The current study included patients treated by microsurgical clipping or endovascular coiling for UIA located in anterior circulation either symptomatic or larger than 3 mm. The cases with complicated UIA such as giant (>25 mm), thrombosed, dissection, or located in the cavernous portion were excluded from the study. The selection of treatment for each case was based on characteristics of individual patients and aneurysms offering both modalities of treatment taking into account patient preference. All clipping and coiling cases were performed on with monitoring of motor evoked potential and somatosensory evoked potential. In clipping cases, the patency of the parent artery, major branches, and visible perforators was confirmed with laser Doppler flowmetry and indocyanine green angiography.
Angiographic results
Angiographic results were divided into complete occlusion, presence of postoperative neck remnant, and re-growth on follow-up. Postoperative computed tomography (CT) images were obtained in the 1st and 7th postoperative days after clipping. In coil embolization, CT was obtained in the 1st postoperative day and magnetic resonance imaging (MRI) in the 5th postoperative day, other follow-up imaging was done at variable intervals depending on the case. Imaging included CT angiography, MRA, and/or digital subtraction angiography [
Ischemic events and clinical outcomes
Ischemic events were defined by the presence of infarction on subsequent CT scan or MRI or angiographic occlusion of a blood vessel by the time of hospital discharge regardless of the presence of symptoms.
The primary outcomes were evaluated as operative complications such as neurological morbidity (irrespective of duration), the presence of postprocedure hemorrhage (subdural, extradural, cerebral, and subarachnoid hemorrhage), or infection (wound infection, meningitis, and pneumonia). The secondary clinical outcome was evaluated by the worsening of the modified Rankin scale (mRS) at discharge.
Independent variables
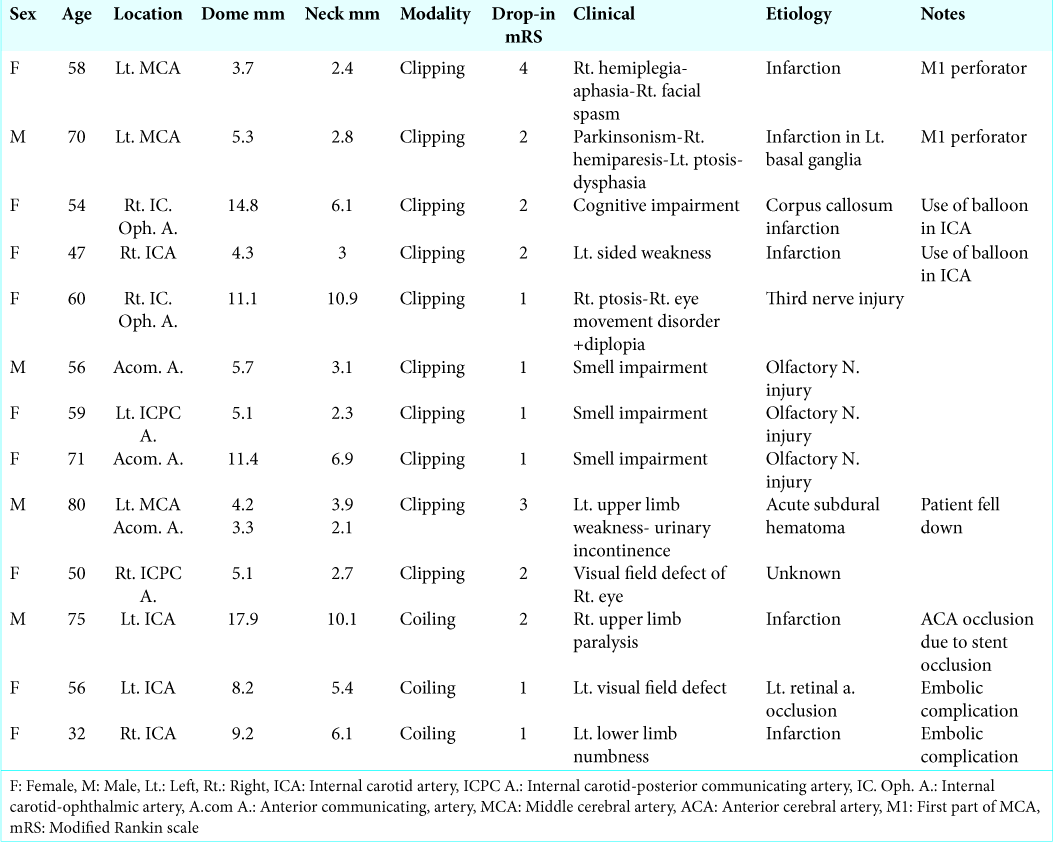
Independent variables investigated as possible risk factors for outcomes included demographic data of patients (age, sex, presence of diabetes mellitus, hyperlipidemia, and hypertension), aneurysm data (location, neck width, dome diameter – parallel to the neck and measured at the largest separation between the aneurysm walls, aneurysm height – the maximum perpendicular distance of the dome from the neck plane, presence of daughter sac, incorporate artery – a branch that is incorporated into the aneurysm wall apart from the normal parent artery, and multiple aneurysms), procedure-related data (temporary arterial occlusion in surgery – the use of balloon and stent in coiling).
Statistics
Categorical variables expressed as a percentile, continuous variables as mean ± SD. Univariate analysis is done using Chi-square test, Fisher’s exact test, and unpaired t-test as appropriate. Multivariate analysis is conducted by multivariate logistic regression and included independent variables that are significant in univariate analysis (P < 0.05), there was only one variable <0.05 in two occasions, in which we considered variables <0.1 for multivariate analysis.
Informed consent
Informed consent is obtained from the patients by the way of the opt-out condition. The present study was approved by the Ethics Committee of Keio University School of Medicine and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
RESULTS
Characteristics of patients and aneurysms
The baseline characteristics of patients and aneurysms are shown in
Ischemic events and clinical outcomes
In this study, the average follow-up duration was 30.2 ± 18.8 months for the surgery group and 29.3 ± 17.6 months for the coiling group. As shown in
The other evaluated factors of outcome are shown in
During surgery, six UIAs were judged as difficult cases to apply the clip and coating was done (two treated later by coiling and four continued under follow-up). In four UIAs, coiling was renounced during the procedure (one is treated by clipping and three continued under follow-up).
Ischemic events were encountered in both groups; the rate of asymptomatic ones is statistically higher in the coiling group (24.5%) than in the clipping group (2%). In contrast, the occurrence rate of symptomatic ischemic complication is almost equal (7.5%) in both groups. Only one case in the coiling group showed both symptomatic and asymptomatic ischemic events.
The occurrence rate of temporary morbidity, including limb paralysis, dysphasia, cognitive dysfunction, cranial nerve affection, CSF leak, diabetes insipidus, and encephalopathy, was significantly higher in the surgery group. The other complications and occurrence rate in each group are also shown in
Predictors for neck remnant and regrowth
Cases, in which clipping and coiling could not be achieved, were excluded from the analysis of postoperative neck remnants. For the surgery group, univariate analysis of risk factors presented the dome diameter >10 mm (P = 0.004), neck width > 4 mm (P = 0.0007), and the presence of incorporate artery (IA) (P = 0.005) to be significant independent variables. Neck width and the presence of IA are significant in multivariate analysis [
For the coiling group, only the presence of IA was significant in univariate analysis (P = 0.02). Multivariate analysis was done with variables <0.1, the presence of IA was significant [
For the regrowth, univariate analysis revealed that the dome diameter >10 mm [P = 0.02], the height of an aneurysm >10 mm (P = 0.02), neck width >4 mm (P = 0.002), and the presence of dome filling after coiling (P = 0.0006) to be significant independent variables, but none was significant in multivariate analysis [
On analysis of cases with ischemic events (symptomatic and asymptomatic) in the surgery group, the height of the aneurysm (> 10 mm), presence of daughter sac, presence of IA, and temporary arterial occlusion were statistically significant predictor by univariate analysis. On multivariate analysis, the presence of IA was the only significant risk for postsurgical ischemic events. There was a strong association between two of these risk factors (height of aneurysm and the presence of daughter sac, P = 0.03).
In the coiling group, the neck width (> 4 mm) is a significant predictor of the ischemic events by univariate analysis. The neck width and location of aneurysm both were statistically significant in multivariate analysis [
The predictors for temporary and permanent morbidity of clipping are shown in
DISCUSSION
The present study demonstrated that small to moderate size UIAs located in anterior circulation were possible to be treated safely by clipping or coiling. The durability was greater in the clipping group than in the coiling group. Although the conclusive outcome was almost even between treatments, the ratio of the ischemic events was higher in the coiling group than the clipping group. In contrast, the risks of specific complications involved in surgical procedure led to the extent of the postoperative hospital period following clipping. We also evaluated the character of UIA as the predictors of some unfavorable postoperative events. These results help to select a more appropriate treatment and suggest the usefulness of hybrid (combination of clipping and coiling) surgery as for UIAs.
Durability
As shown in our data, the lower risk of neck remnant and retreatment is thought to be an advantage of clipping comparing to coiling. This result is in line with the previous reports.[
On the contrary, our presented higher regrowth rate after coil embolization is also in line with other studies.[
Ischemic events
In our study, symptomatic ischemic events occurred in 11 cases (7.5%) and the neurological deterioration persisted in 4 cases (2.7%) after clipping. The incidence of cerebral infarction was reported to be 11–12% after clipping.[
Regarding M1 perforator obstruction, both aneurysms were of a small size and located on M1 trunk with superior projection. In the previous literature, M1 perforator injury was more frequently associated with superior projection aneurysms.[
During clipping with craniotomy, the systemic enough heparinization to prevent thrombotic events induced by balloon occlusion of the parent artery is difficult because of bleeding during surgery. Although there is a cosmetic problem, proximal flow control of the parent artery with direct exposure of the cervical carotid artery is a considerable method to avoid ischemic complications.
In our coiling group, symptomatic ischemic events occurred in 4 cases (7.5%) and the neurological deterioration persisted in 3 cases (5.6%). Thromboembolic events are the most encountered complication in the endovascular treatment of aneurysms.[
Functional outcome and morbidity
In the current study, the rate of the patients with mRS >2 was 1.3% in clipping and 0% in coiling. Whereas it showed no statistical significance, better outcome was obtained in the coiling group than in the clipping group. Moreover, the length of hospital stay was significantly longer in clipping than coiling. Hoh et al. and Darsaut et al. have documented the similar trends related to hospitalization period.[
The rate of permanent morbidity in the current study was 5.6% and 6.8% after coiling and clipping, respectively. It was not possible to analyze the predictors for this low number of cases in the coiling group, while the presence of multiple aneurysms and temporary parent artery occlusion with a balloon was significant predictors of post clipping permanent morbidity. Orz et al. demonstrated the risk of multiple aneurysms for the unfavorable functional outcome.[
Cases with postoperative permanent morbidity in endovascular treatment were all related to thromboembolic events, all of them possess a wide neck more than 4 mm, a significant risk factor for thromboembolic events is identified in the current study. Cases with such a risk factor should have proper antiplatelet management with strict preoperative and postoperative monitoring of patient response to antiplatelet therapy.
Limitations
This study has some limitations, first, with the retrospective study reviewed from medical records only. Surgical indication and treating methods were determined by neurosurgeon individually. Secondary, the refinement of endovascular devices associated with a better outcome was not fully considered. The technique of clipping has been established well by expert surgeons, while the endovascular treatment for UIA has continued to develop along with the advancement of devices. Third, the follow-up period was short to evaluate the regrowth and rupture of UIA in the future. Therefore, further investigation in a large-scale RCT for its durability and long-term follow-up is warranted to help physicians when determining an appropriate decision in the treatment of UIAs.
CONCLUSION
As the treatment for UIA, the clipping and endovascular coiling were safe and feasible. The clipping was advantageous in durability, while the rate of morbidity was lower and the hospitalization period was shorter in the coiling group. It is appropriate for Japan to follow the recent world trend in coiling. Nonetheless, we must consider a system that inherits the skills of the clipping. The clipping and coiling should coexist while complementing each other by understanding the advantages and disadvantages of both.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahn SS, Kim YD. Three-dimensional digital subtraction angiographic evaluation of aneurysm remnants after clip placement. J Korean Neurosurg Soc. 2010. 47: 185-90
2. Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Unruptured cerebral aneurysms: Evaluation and management. Scientific World Journal. 2015. 2015: 954954-
3. Alshafai N, Cusimano MD, Falenchuk O. Global differences in the present and future management of cerebral aneurysms. World Neurosurg. 2013. 80: 717-22
4. Blackburn SL, Abdelazim AM, Cutler AB, Brookins KT, Fargen KM, Hoh BL. Endovascular and surgical treatment of unruptured MCA aneurysms: Meta-analysis and review of the literature. Stroke Res Treat. 2014. 2014: 348147-
5. Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ. Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001-2008. AJNR Am J Neuroradiol. 2011. 32: 1071-5
6. Brown MA, Parish J, Guandique CF, Payner TD, Horner T, Leipzig T. A long-term study of durability and risk factors for aneurysm recurrence after microsurgical clip ligation. J Neurosurg. 2017. 126: 819-24
7. Darsaut TE, Findlay JM, Magro E, Kotowski M, Roy D, Weill A. Surgical clipping or endovascular coiling for unruptured intracranial aneurysms: A pragmatic randomised trial. J Neurol Neurosurg Psychiatry. 2017. 88: 663-8
8. Feng Y, Wada S, Tsubota K, Yamaguchi T. A model-based numerical analysis in the early development of intracranial aneurysms. Conf Proc IEEE Eng Med Biol Soc. 2005. 1: 607-10
9. Gerlach R, Beck J, Setzer M, Vatter H, Berkefeld J, Du Mesnil de Rochemont R. Treatment related morbidity of unruptured intracranial aneurysms: Results of a prospective single centre series with an interdisciplinary approach over a 6 year period (1999-2005). J Neurol Neurosurg Psychiatry. 2007. 78: 864-71
10. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
11. Hoh BL, Chi YY, Lawson MF, Mocco J, Barker FG. Length of stay and total hospital charges of clipping versus coiling for ruptured and unruptured adult cerebral aneurysms in the nationwide inpatient sample database 2002 to 2006. Stroke. 2010. 41: 337-42
12. Iwama T, Yoshimura S, Kaku Y, Sakai N. Considerations in the surgical treatment of superior-wall type aneurysm at the proximal (M1) segment of the middle cerebral artery. Acta Neurochir (Wien). 2004. 146: 967-72
13. Jabbarli R, Pierscianek D, Wrede K, Dammann P, Schlamann M, Forsting M. Aneurysm remnant after clipping: The risks and consequences. J Neurosurg. 2016. 125: 1249-55
14. Jang EW, Kim YB, Chung J, Suh SH, Hong CK, Joo JY. Clinical risk factors affecting procedure-related major neurological complications in unruptured intracranial aneurysms. Yonsei Med J. 2015. 56: 987-92
15. Kawabata Y, Nakazawa T, Fukuda S, Kawarazaki S, Aok T, Morita T. Endovascular embolization of branch-incorporated cerebral aneurysms. Neuroradiol J. 2017. 30: 600-6
16. Kim J, Chang C, Jung Y. Feasibility and midterm outcomes of endovascular coil embolization of an unruptured middle cerebral artery aneurysm with an incorporated branch. World Neurosurg. 2018. 118: e745-52
17. Layton KF, Cloft HJ, Gray LA, Lewis DA, Kallmes DF. Balloon-assisted coiling of intracranial aneurysms: Evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol. 2007. 28: 1172-5
18. Li M, Wu J, Chen X, Jiang P, Yang F, Ma Y. Symptomatic and silent cerebral infarction following surgical clipping of unruptured intracranial aneurysms: Incidence, risk factors, and clinical outcome. Neurosurg Rev. 2018. 41: 675-82
19. McDonald JS, McDonald RJ, Fan J, Kallmes DF, Lanzino G, Cloft HJ. Comparative effectiveness of unruptured cerebral aneurysm therapies: Propensity score analysis of clipping versus coiling. Stroke. 2013. 44: 988-94
20. Murayama Y, Takao H, Ishibashi T, Saguchi T, Ebara M, Yuki I. Risk analysis of unruptured intracranial aneurysms: Prospective 10-year cohort study. Stroke. 2016. 47: 365-71
21. Orz YI, Hongo K, Tanaka Y, Nagashima H, Osawa M, Kyoshima K. Risks of surgery for patients with unruptured intracranial aneurysms. Surg Neurol. 2000. 53: 21-7
22. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003. 34: 1398-403
23. Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J. Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol. 2007. 28: 1755-61
24. Schirmer CM, Malek AM. Computational fluid dynamic characterization of carotid bifurcation stenosis in patient-based geometries. Brain Behav. 2012. 2: 42-52
25. Seo DH, Yoon SM, Park HR, Shim JJ, Bae HG, Yun IG. Thromboembolic event detected by diffusion weighted magnetic resonance imaging after coil embolization of cerebral aneurysms. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 175-83
26. Shigematsu T, Fujinaka T, Yoshimine T, Imamura H, Ishii A, Sakai C. Endovascular therapy for asymptomatic unruptured intracranial aneurysms: JR-NET and JR-NET2 findings. Stroke. 2013. 44: 2735-42
27. Soeda A, Sakai N, Sakai H, Iihara K, Yamada N, Imakita S. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: Evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2003. 24: 127-32
28. Tominari S, Morita A, Ishibashi T, Yamazaki T, Takao H, Murayama Y. Prediction model for 3-year rupture risk of unruptured cerebral aneurysms in Japanese patients. Ann Neurol. 2015. 77: 1050-9
29. van Rooij WJ, Sluzewski M, Beute GN, Nijssen PC. Procedural complications of coiling of ruptured intracranial aneurysms: Incidence and risk factors in a consecutive series of 681 patients. AJNR Am J Neuroradiol. 2006. 27: 1498-501
30. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003. 362: 103-10
31. Yeon JY, Kim JS, Hong SC. Angiographic characteristics of unruptured middle cerebral artery aneurysms predicting perforator injuries. Br J Neurosurg. 2011. 25: 497-502