- Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States.
- Department of Neurology University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States.
- Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States.
Correspondence Address:
Andrew K. Conner
Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, United States.
DOI:10.25259/SNI_867_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jan Bian1, Alison Westrup1, Sarah Sung1, Nidhiben A. Anadani2, Kar-Ming Fung3, Andrew K. Conner1. Co-existence of multiple sclerosis and germinoma in an adult male: Case report. 19-Apr-2021;12:177
How to cite this URL: Jan Bian1, Alison Westrup1, Sarah Sung1, Nidhiben A. Anadani2, Kar-Ming Fung3, Andrew K. Conner1. Co-existence of multiple sclerosis and germinoma in an adult male: Case report. 19-Apr-2021;12:177. Available from: https://surgicalneurologyint.com/surgicalint-articles/10730/
Abstract
Background: Concurrent diagnosis of multiple sclerosis (MS) and the central nervous system (CNS) germinoma is rare. The diagnostic criteria for MS rely primarily on clinical presentation, and CNS germinoma can present as an MS mimic. These factors contribute to the rarity of dual diagnosis.
Case Description: A 28-year-old man presented initially with bilateral optic neuritis, manifesting as persistently worsening vision for 2 years, and demyelinating plaques identified within the corpus callosum on magnetic resonance imaging. Initial work-up, in addition to clinical presentation, led to diagnosis of MS. Three months following the diagnosis of MS, the patient then presented with obstructive hydrocephalus due to a newly diagnosed intraventricular mass. The patient underwent an endoscopic third ventriculostomy and biopsy which confirmed diagnosis of CNS germinoma.
Conclusion: To the best of our knowledge, dual presentation of both MS and CNS germinoma has never been reported in the literature. The clinical presentation of bilateral optic neuritis (persisting for roughly 2 years before initial MS diagnosis), demyelinating plaques, and intrathecal oligoclonal bands before the development of an intraventricular mass indicates that both MS and CNS germinoma presented simultaneously in this patient. The treatment plan for this patient included carboplatin + etoposide, followed by adjuvant radiation and subsequent IVIG therapy.
Keywords: Dawson’s fingers, Endoscopic third ventriculostomy, Germinoma, Multiple sclerosis
INTRODUCTION
Primary intracranial germinomas are derived from germinal stem cells and are most often located in the suprasellar or infundibular, and pineal regions of the brain.[
A few cases have been described in the literature, in which CNS germinomas were originally diagnosed as MS. This misdiagnosis occurs because detection of these diseases relies mostly on clinical symptoms coupled with neuroimaging, and both can present with abnormal brain MRI.[
CASE PRESENTATION
Clinical presentation
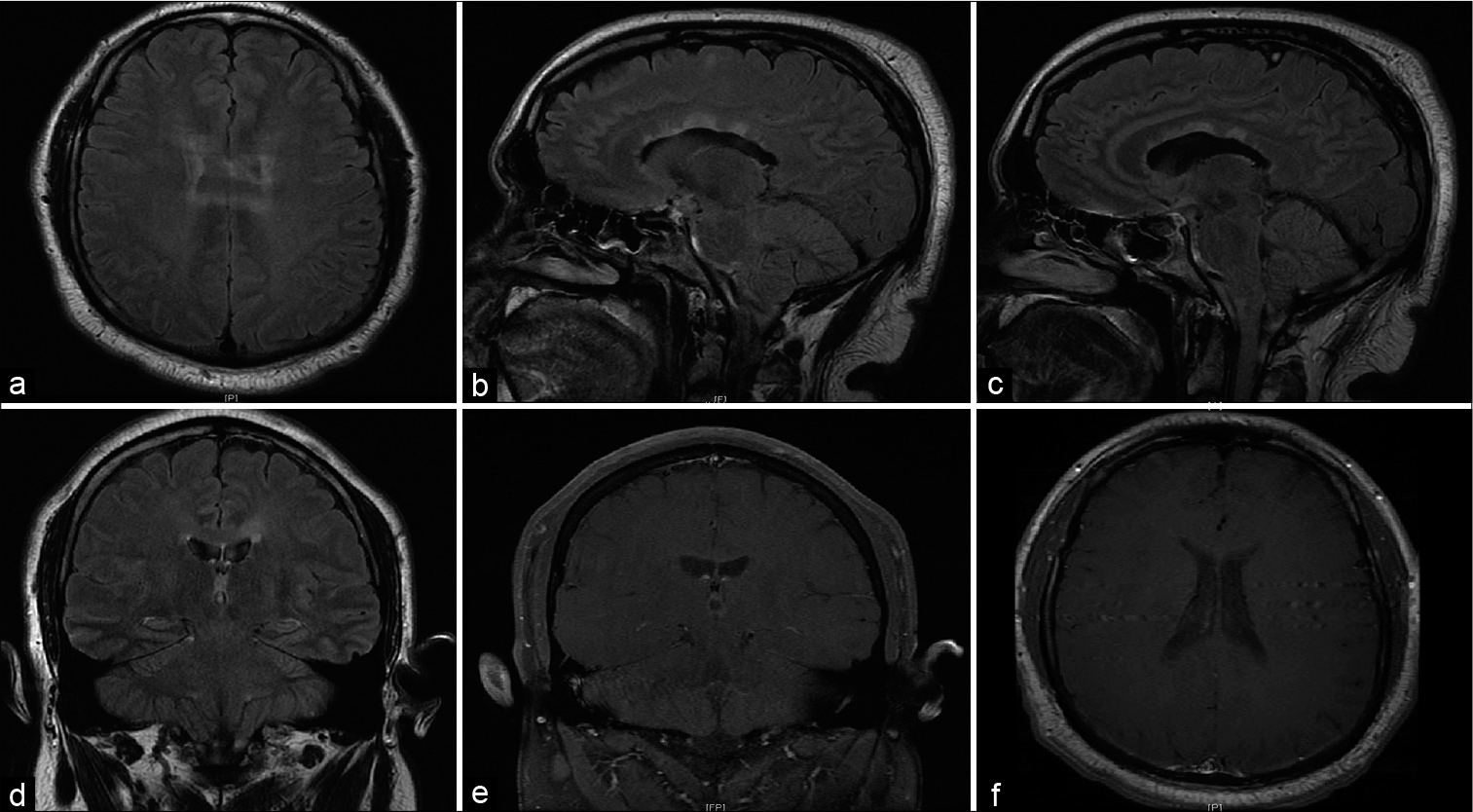
The patient described is a 28-year-old man with a history of hypertension who initially presented to the ophthalmology department with a 2-year history of progressive, bilateral vision loss. Findings on exam included poor visual acuity, bilateral optic atrophy, reduced foveal threshold, and nonspecific inferior defects. Fundoscopic exam at this time revealed optic disc pallor and a cup-to-disc ratio of 0.3 bilaterally. Given these findings, the patient was subsequently referred to the department of neurology for evaluation of possible MS. Brain MRI revealed T2 FLAIR hyperintense lesions involving the body of the corpus callosum and the periventricular white matter without contrast enhancement, suggestive of demyelinating plaques [
Figure 1:
(a) Axial view showing corpus callosum lesions (b and c) sagittal T2 FLAIR images showing corpus callosal lesions and periventricular lesions oriented perpendicular to long axis of lateral ventricle (d) ovoid periventricular lesion (e and f) coronal T1 + contrast and axial T1 + contrast demonstrating absence of lesions.
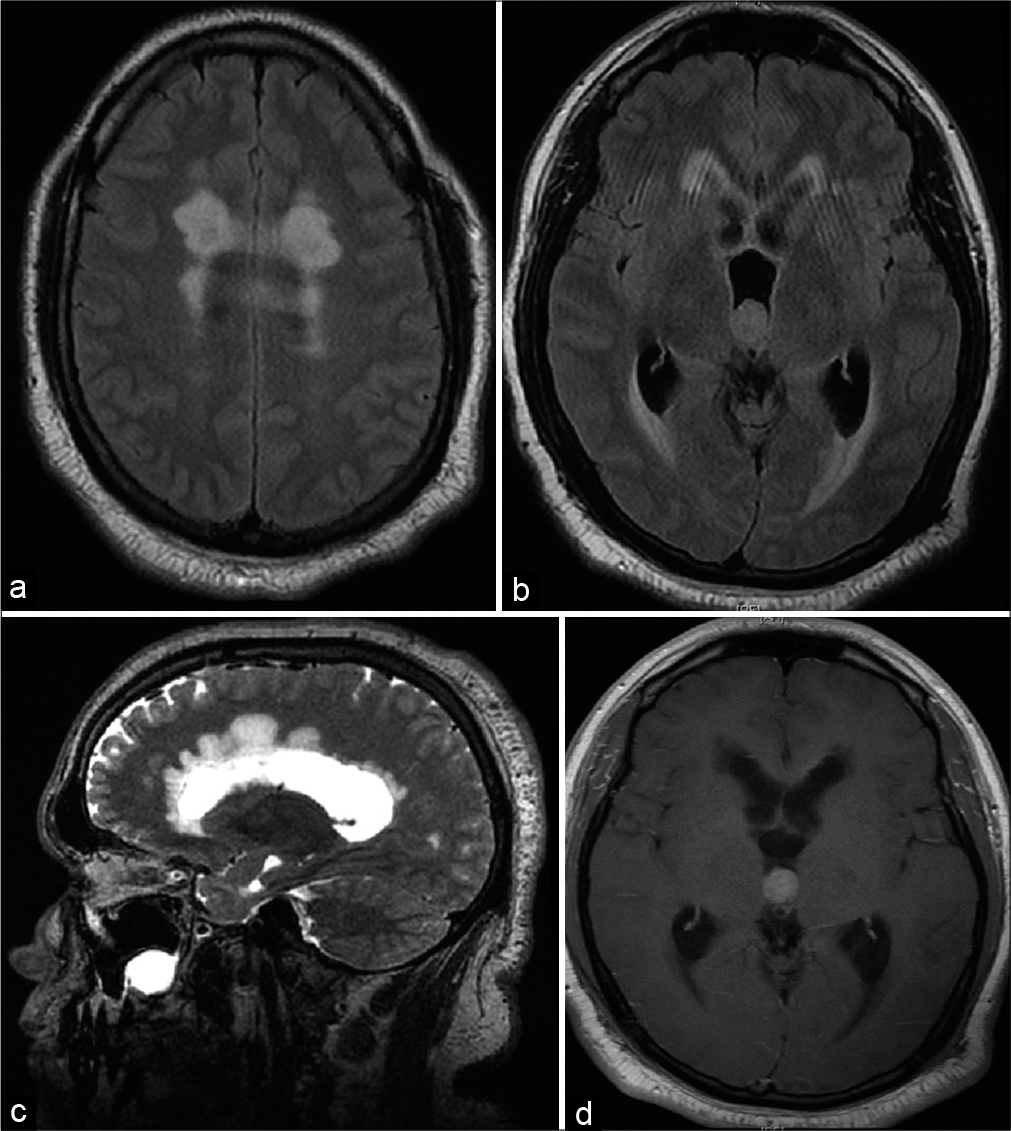
Three months after the diagnosis of MS, the patient presented to an outside hospital with a severe right-sided headache and he was referred to our hospital for further evaluation secondary to imaging demonstrating hydrocephalus and intracranial mass. Of note, the patient had not begun treatment for MS at the time of his hospital admission. At time of presentation, the patient was alert and in no apparent distress. The patient characterized the headache as constant for the past month, and worsened in the week before presentation. He denied any vomiting or nausea and his neurological examination was unremarkable. No upper motor neuron findings or visual field deficits were present at the time of examination. Fundoscopic exam showed no relative afferent pupillary defect. MRI of his brain demonstrated an intraventricular mass located in the posterior third ventricle, obstructive hydrocephalus and worsening of corpus callosum and periventricular T2 hyperintense lesions [
Figure 2:
(a) Axial T2 FLAIR showing worsening of previously noted corpus callosum lesion (b) axial T2 FLAIR showing an ovoid 3rd ventricular mass, obstructive hydrocephalus, and periventricular T2 hyperintensity (c) sagittal T2 image showing worsening of previously noted periventricular lesions (d) T1 axial post contrast scan showing homogenous enhancement of 3rd ventricle mass.
Pathology
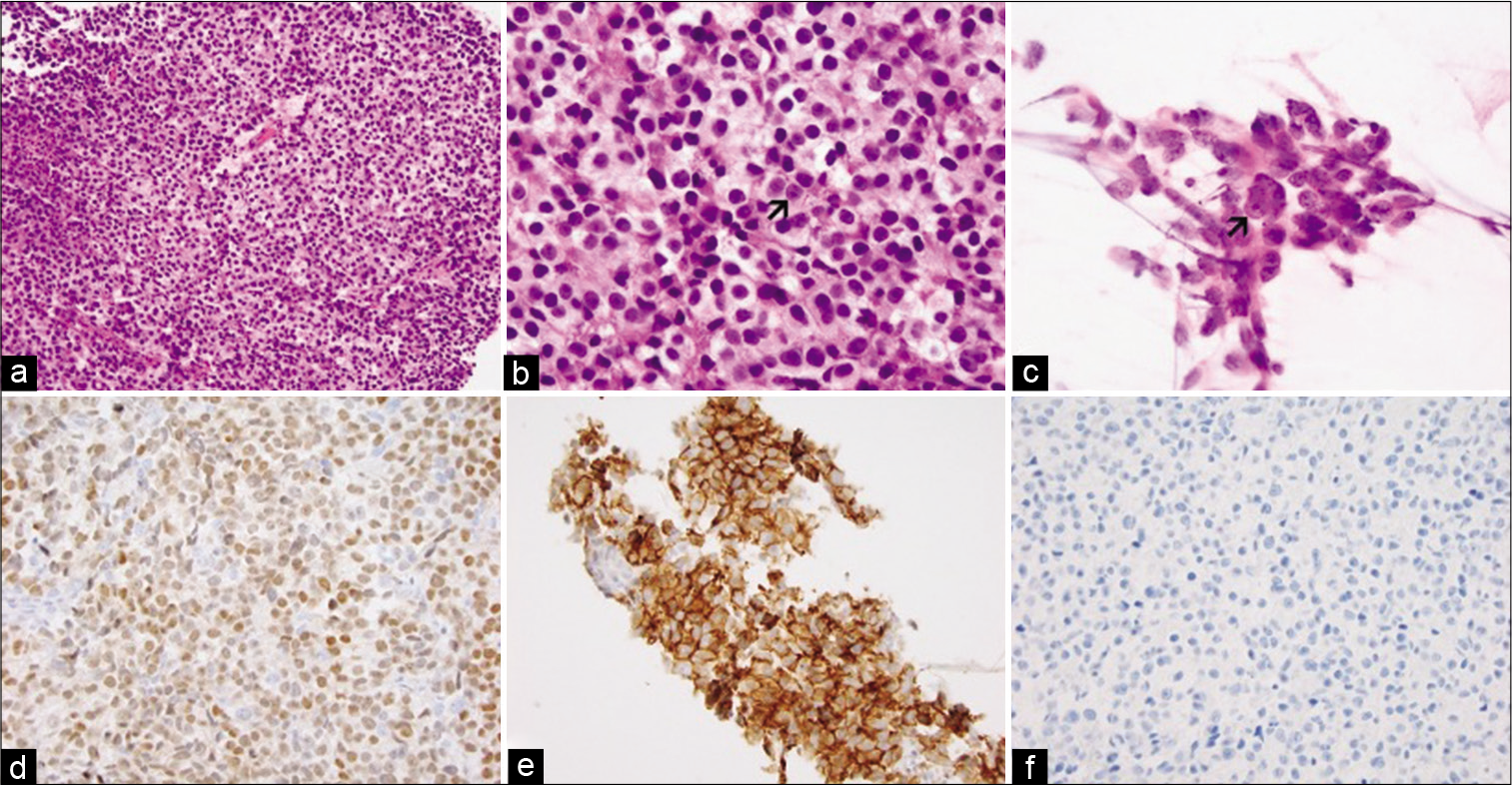
Two small biopsy specimens were submitted for examination. The tumor contained solid sheets of polygonal cells of moderate size and centrally located nuclei. The cytoplasm was pale in some areas [
Figure 3:
(a and b) are low- and high-magnification. (c) is cytologic preparation during intraoperative consultation. Prominent nucleoli are highlighted by arrows in (b and c). (d-f) are immunohistochemistry for OCT3/4, CD117, and H3 K27M respectively. Original magnification for (a) is ×20, (b) and (c) are ×60, (d) to (f) are ×40.
Postoperative course
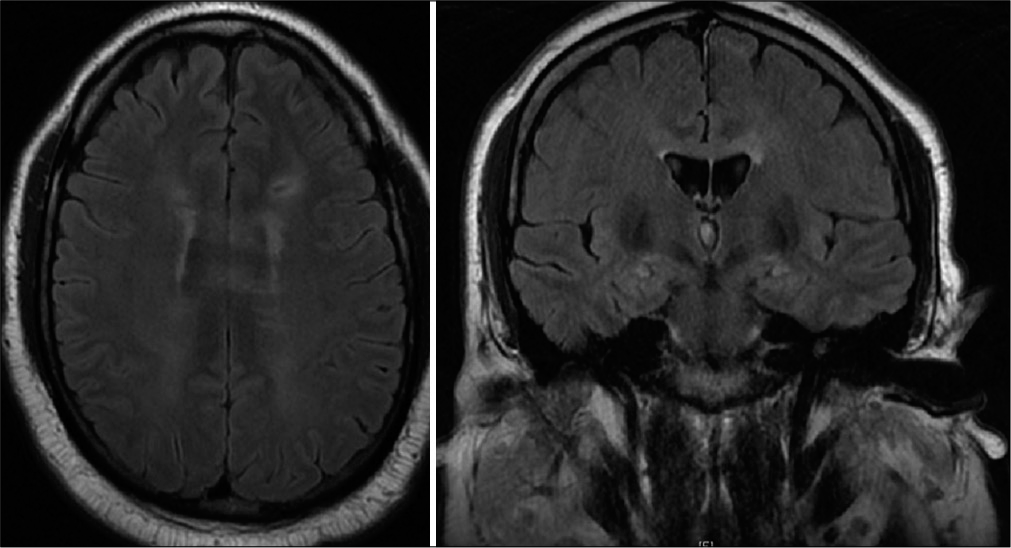
The patient’s neurologic exam was normal postoperatively and he was discharged home after a 2-day hospital stay. Two months after surgery, the patient was started on carboplatin + etoposide to attempt cytoreduction before radiation therapy to treat intracranial germinoma. Interestingly, brain MRI 4 months after surgery showed resolution of hydrocephalus, periventricular T2 hyperintense changes (thought to possibly be related to trans-ependymal flow initially) and decrease in size of corpus callosum plaques [
DISCUSSION
Primary intracranial germinomas generally present with clinical symptoms that include headaches and vision changes and are definitively diagnosed with an abnormal MRI and brain mass biopsy.[
The patient’s presentation with a clinically isolated syndrome of bilateral optic neuritis, lesional demyelination on MRI, and intrathecal oligoclonal bands all fulfill the McDonald criteria for an MS diagnosis.[
Paraneoplastic optic neuritis induced by the CNS germinoma was considered as an explanation for the patient’s bilateral optic neuritis. This rare, autoimmune reaction can cause optic neuropathy in CNS tumors.[
There have been a few reports where intrathecal oligoclonal bands were reported in germinoma patients.[
The treatment plan for this patient focused on addressing the CNS germinoma first before managing the MS. The patient completed four cycles of carboplatin + etoposide with near complete response of the ventricular germinoma and no evidence of disease in the spine. Shortly thereafter, the patient is slated to begin radiation therapy. He is scheduled to start IVIG therapy following radiation treatment which has been shown to slow vision loss in MS.[
At the time of writing this manuscript, the patient’s headaches have improved significantly since completion of chemotherapy. Future IVIG therapy will hopefully slow vision loss due to optic neuritis caused by MS.
CONCLUSION
Concurrent presentation of MS and CNS germinoma is exceptionally rare. However, after careful consideration of the clinical presentation of our patient, combined with imaging and biopsy, a final dual diagnosis was made. Development of a treatment plan for coexisting MS and CNS germinoma is complex, but the emphasis was ultimately placed first on treating the CNS germinoma, to be followed with targeted MS therapy. For now, the current treatment appears to have addressed the patient’s tumor-related symptoms. Future radiation and IVIG therapy will hopefully prevent further vision loss due to MS.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Achiron A. Winning combination: The additive/synergistic benefits of IVIg in corticosteroid refractory optic neuritis. Eur J Neurol. 2008. 15: 1145
2. Akiyama Y, Suzuki H, Mikuni N. Germinoma mimicking tumefactive demyelinating disease in pediatric patients. Pediatr Neurosurg. 2016. 51: 149-53
3. Kageyama H, Suzuki T, Ohara Y. Intramedullary spinal cord germinoma clinically mimicking multiple sclerosis: A case report. Surg Neurol Int. 2019. 10: 201-1
4. Krolak-Salmon P, Androdias G, Honnorat J, Caudie C, Bret P, Hernette D. Beware of optic neuritis! Lancet Neurol. 2002. 1: 516-7
5. Oh J, Vidal-Jordana A, Montalban X. Multiple sclerosis: Clinical aspects. Curr Opin Neurol. 2018. 31: 752-9
6. Osorio DS, Allen JC. Management of CNS germinoma. CNS Oncol. 2015. 4: 273-9
7. Shi-Yun C, Yue-Shan P, De-Hong LU. Intracranial germinoma with ventricular system dissemination. Chin J Contemp Neurol Neurosurg. 2014. 14: 416-20
8. Strowd RE, Burger P, Holdhoff M, Kleinberg L, Okun MS, Olivi A. Steroid-responsive intracranial germinoma presenting as Holmes’ tremor: Importance of a tissue diagnosis. J Clin Neurosci. 2015. 22: 911-3
9. Thakkar JP, Chew L, Villano JL. Primary CNS germ cell tumors: Current epidemiology and update on treatment. Med Oncol. 2013. 30: 496-6
10. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018. 17: 162-73
11. Xu Q, Du W, Zhou H, Zhang X, Liu H, Song H. Distinct clinical characteristics of paraneoplastic optic neuropathy. Br J Ophthalmol. 2019. 103: 797-801