- Department of Neurosurgery, Lenox Hill Hospital, New York, NY, United States,
- Department of Neurosurgery, Kochi University Hospital, Kochi, Japan,
- Department of Surgery, Division of Neurosurgery, Queen Mary Hospital, University of Hong Kong, Hong Kong,
- Department of Neurosurgery, Faculty of Medicine, The University of Tokyo, Bunkyo-ku, Tokyo,
- Department of Neurosurgery, Hyogo Prefectural Amagasaki General Medical Center, Kyoto University, Amagasaki City, Hyogo, Japan.
Correspondence Address:
Benjamin W. Y. Lo
Department of Surgery, Division of Neurosurgery, Queen Mary Hospital, University of Hong Kong, Hong Kong,
DOI:10.25259/SNI_242_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Benjamin W. Y. Lo1, Hitoshi Fukuda2, Anderson C. O. Tsang3, David J. Langer1, Satoru Miyawaki4, Masaomi Koyanagi5, Matthew Wai-Man Lui3. Commentary on Post, et al. Ultra-early tranexamic acid after subarachnoid hemorrhage: A randomized controlled trial. Lancet 2021. 14-Apr-2021;12:156
How to cite this URL: Benjamin W. Y. Lo1, Hitoshi Fukuda2, Anderson C. O. Tsang3, David J. Langer1, Satoru Miyawaki4, Masaomi Koyanagi5, Matthew Wai-Man Lui3. Commentary on Post, et al. Ultra-early tranexamic acid after subarachnoid hemorrhage: A randomized controlled trial. Lancet 2021. 14-Apr-2021;12:156. Available from: https://surgicalneurologyint.com/surgicalint-articles/10716/
Abstract
Background: Tranexamic acid (TA) administration in aneurysmal subarachnoid hemorrhage (SAH) within the first 24 hours may reduce the incidence of early aneurysmal rebleeding. However, this is also the potential for an increased risk of delayed cerebral ischemia if TA is administered for more than 72 hours following the initial aneurysmal rupture.
Methods: In the ultra-early tranexamic acid after subarachnoid hemorrhage randomized controlled trial by Post et al., patients were randomized to receive TA within the first 24 hours, or until start of aneurysm treatment. These results were compared to a matched control group.
Results: Ultra-early administration (≤24 h) of TA reduced the incidence of rebleeding, and did not alter the incidence of delayed cerebral ischemia and/or extracranial thrombosis. Further, no significant differences were noted between the TA group and control arm in the incidence of good (modified Rankin scores 0-3) clinical outcomes at 6 months.
Conclusion: Ultra-early administration of TA (≤24 h) resulted in a lower rate of recurrent hemorrhage, without increasing the incidence of delayed cerebral ischemia in SAH patients.
Keywords: Randomized controlled trial, Subarachnoid hemorrhage, Tranexamic acid,
INTRODUCTION
Cerebral aneurysmal re-rupture in subarachnoid hemorrhage (SAH) most frequently occurs within the first 6 h and is most likely due to antithrombotic therapy, hypertension, coagulopathy, Valsalva maneuvers, multisystemic, and metabolic disturbances. Recently, we have focused on delineating the potential benefits of “early (i.e.
TA – PHYSIOLOGICAL ACTION
TA administration in SAH patients has multiple physiological benefits as well as risks. TA forms a reversible complex that displaces plasminogen from fibrin, resulting in inhibition of both fibrinolysis and proteolytic activity of plasmin; decreasing the potential for aneurysmal rebleeding. However, possible side effects of TA may include increasing the risk of delayed cerebral ischemia if administered beyond 72 hours following the initial aneurysmal rupture [
ULTRA-EARLY TRANEXAMIC ACID AFTER SUBARACHNOID HEMORRHAGE (ULTRA) TRIAL
Post et al. performed an open-label randomized controlled trial (RCT) that involved eight treatment centers, and 16 referral hospitals in the Netherlands (2013–2019).[
TIMING OF ADMINISTRATION OF TA
Patients randomized to the TA treatment arm received therapy within an average of 3 hours following admission, after initial CT confirmation of their SAH diagnosis. The median time to aneurysm treatment after CT diagnosis was 14 hours (IQR 5–20 hours).
OUTCOMES
Sixty-percent of TA patients versus 64% of control group patients had good 6-month clinical outcomes (modified Rankin scores 0–3). However, 48% of TA patients versus 56% of control group patients had excellent 6-month outcomes (modified Rankin scores 0–2) [
IMPACT OF ULTRA-EARLY TA ADMINISTRATION
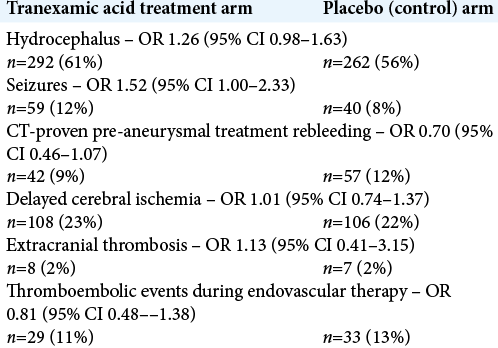
Ultra-early TA administration (≤24 h) resulted in a lower rate of aneurysmal rebleeding prior to definitive aneurysmal treatment. Notably, however, it did not predispose patients to more thrombotic complications during endovascular therapy, nor did it increase the incidence of delayed cerebral ischemia and/or extracranial thrombosis [
CONCLUSION
This RCT, by Post et al. demonstrated that ultra-early administration of TA in aneurysmal SAH for maximum of 24 hours did not alter the incidence of delayed cerebral ischemia or extracranial thrombosis.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baharoglu MI, Germans MR, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2013. 8: CD001245
2. Post R, Germans MR, Tjerkstra MA, Vergouwen MD, Jellema K, Koot RW. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): A randomised controlled trial. Lancet. 2021. 397: 112-8