- Department of Neurosurgery, Kurume University School of Medicine, Fukuoka, Japan
Correspondence Address:
Takachika Aoki
Department of Neurosurgery, Kurume University School of Medicine, Fukuoka, Japan
DOI:10.4103/2152-7806.187492
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Aoki T, Hirohata M, Noguchi K, Komaki S, Orito K, Morioka M. Comparative outcome analysis of anterior choroidal artery aneurysms treated with endovascular coiling or surgical clipping. Surg Neurol Int 01-Aug-2016;7:
How to cite this URL: Aoki T, Hirohata M, Noguchi K, Komaki S, Orito K, Morioka M. Comparative outcome analysis of anterior choroidal artery aneurysms treated with endovascular coiling or surgical clipping. Surg Neurol Int 01-Aug-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/comparative-outcome-analysis-anterior-choroidal-artery-aneurysms-treated-endovascular-coiling-surgical-clipping/
Abstract
Background:Treatment of anterior choroidal artery (AChA) aneurysms with endovascular coiling or surgical clipping may increase the risk of ischemic complications owing to the critical territory supplied by the AChA. We analyzed the surgical results of endovascular coiling and surgical clipping for AChA aneurysms performed in a single institution, as well as the role of indocyanine green-videoangiography (ICG-VAG) and motor-evoked potential (MEP).
Methods:We analyzed 50 patients (51 aneurysms; 21 men, 29 women; mean age: 58 years) including 25 with subarachnoid hemorrhage treated with endovascular coiling or surgical clipping between April 1990 and October 2013. The complication rates and clinical outcomes of the coil group (mean follow-up: 61 months) and the clip group (mean follow-up: 121 months) were analyzed with a modified Rankin scale.
Results:The overall clinical outcome of the coil group (95%) was better than that of the clip group (85%). Especially, the outcomes in the coil group were better in the first investigated period (1990–2007) (P P = 0.005), and treatment-related complications decreased from 20 to 4.7%. Eleven aneurysms (coil group: 8, clip group: 3) showed small neck remnants but no remarkable regrowth, except for 1 case during the mean follow-up period of 91 months.
Conclusions:Surgical clipping of AChA aneurysms has become safer because of ICG-VAG and MEP monitoring. Coiling and clipping of AChA aneurysms showed good and comparable outcomes with these monitoring methods.
Keywords: Anterior choroidal artery aneurysm, endovascular coiling, surgical clipping
INTRODUCTION
Anterior choroidal artery (AChA) aneurysms are rare, representing 2–5% of all intracranial aneurysms. Furthermore, few literature reports have focused on microneurosurgical treatment.[
MATERIALS AND METHODS
Patients
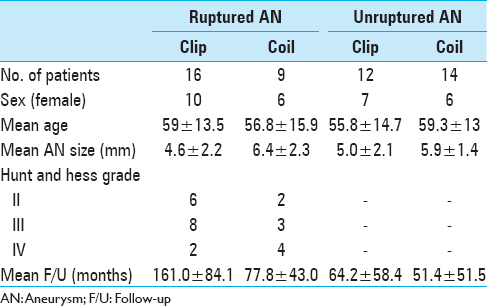
We performed a retrospective analysis of 50 patients (51 AChA aneurysms) who were treated with endovascular coiling or surgical clipping at the Kurume University School of Medicine between April 1990 and October 2013. Of these patients, 21 were men and 29 were women, with a mean age of 58.0 years. The average aneurysm size was 5.5 mm (range, 2–10.1 mm). The study participants included 25 patients with subarachnoid hemorrhage (SAH). In these patients, the distribution of Hunt and Hess grades II, III, and IV was 8, 11, and 6, respectively [
Selection of treatment method: Endovascular coiling or surgical clipping
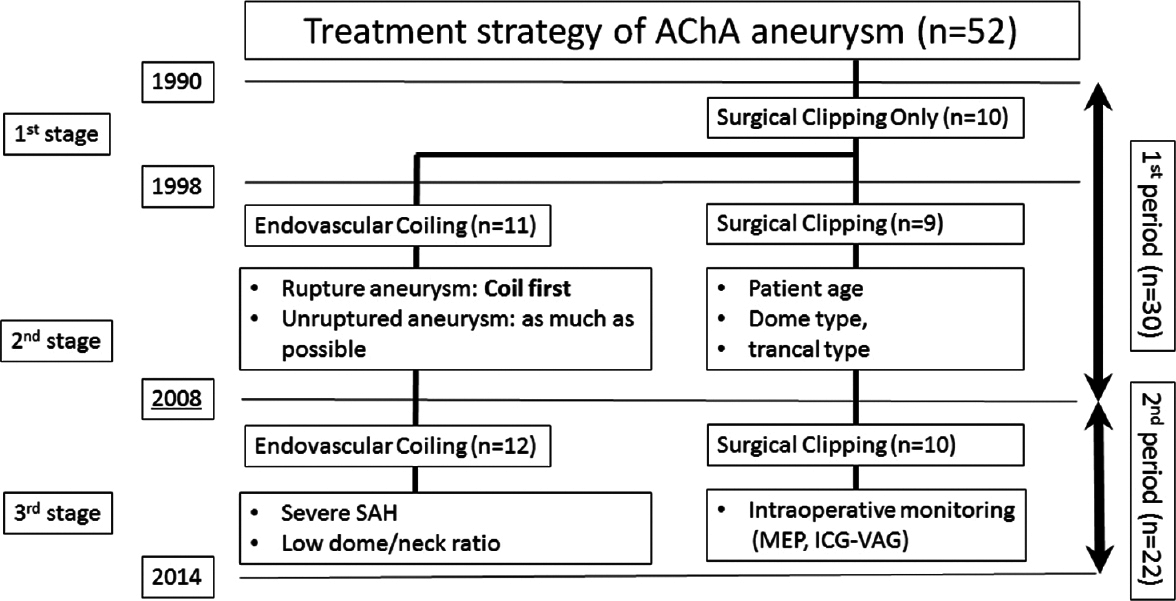
The fundamental treatment strategy of our department for AChA aneurysms has changed over time. During the first examined period (1990–1997), surgical clipping was the preferred method. However, during the second period (1998–2007), endovascular coiling became the first choice owing to the incidence of AChA occlusion after clipping and the subsequent development of an endovascular coiling device. During the third period (2008–present), with the introduction of ICG-VAG and MEP, the treatment method of choice has been based on the circumstances of each case; endovascular coiling has become the first choice for patients with severe SAH (Hunt and Hess grades III and IV and/or small neck aneurysm) with an appropriate neck-to-dome ratio (<1.5). However, other cases have required surgical clipping with ICG-VAG and MEP monitoring.
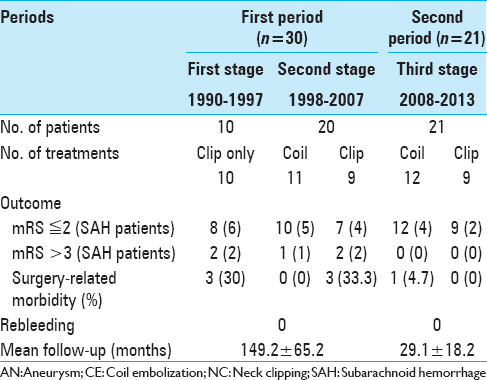
In this study, we evaluated the clinical outcomes of patients treated within the first and second periods combined, and those treated during the third period [
Surgical complications and clinical outcomes
In this study, surgical complications were defined as new neurological deficits lasting 24 h or longer—after treatment with coil embolization or surgical clipping—that were associated with a new infarction or hemorrhage on magnetic resonance imaging or computed tomography (excluding vasospasms on SAH). The clinical outcomes were evaluated with a modified Rankin scale (mRS) during the last follow-up [
Classification of anterior choroidal artery aneurysms in this study
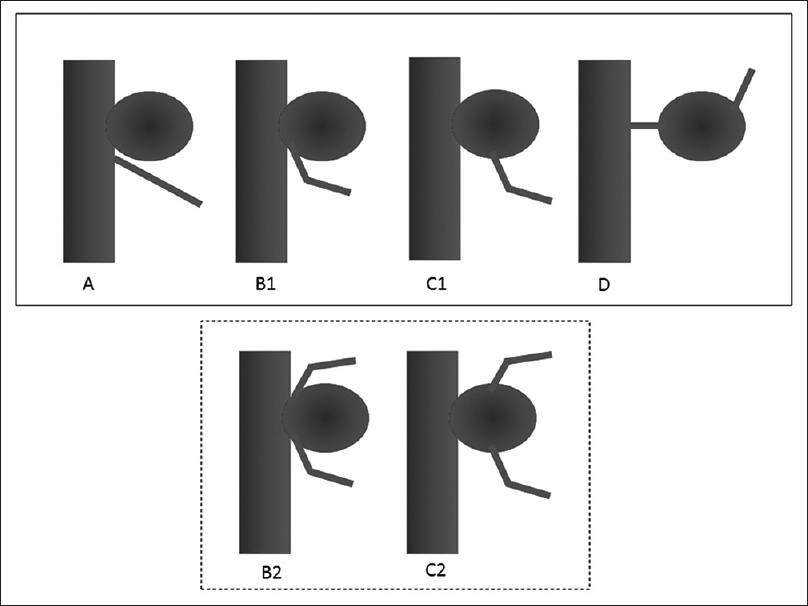
We have devised a new classification—representing a modification of the one used in previous reports[
Figure 2
Classification of anterior choroidal artery (AChA) aneurysm types. A, Artery type—AChA arises from the internal carotid artery. B1, Neck type—AChA arises from the aneurysmal neck. B2, Neck subtype—Aneurysm is located between the duplicated AChAs. C1, Dome type—AChA arises from the aneurysmal dome. C2, Dome subtype—Duplicated AChA arises from the aneurysmal dome. D, Truncal type—Aneurysm originates in a portion of the AChA itself
RESULTS
Overall outcomes
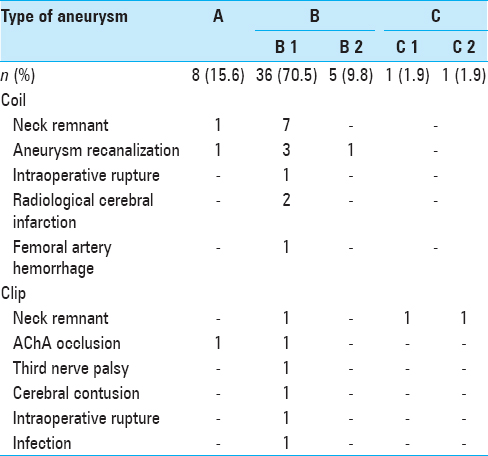
The overall outcomes are listed in
According to our aneurysm type classification [
Clinical outcomes in the coil group
Twenty-two patients in the coil group (95.6%) had favorable outcomes (mRS ≤ 2) including 9 patients with SAH [
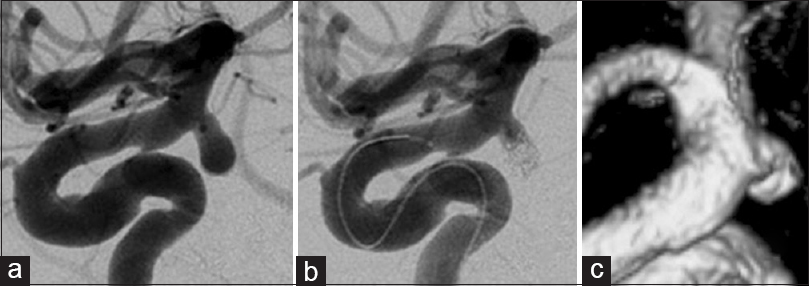
Figure 3
Findings in a 79-year-old woman (a) Preoperative angiogram shows the right anterior choroidal artery (AChA) with a ruptured aneurysm. The AChA arises from the aneurysmal neck (classified as type B1). (b) Endovascular coil embolization is performed with small neck remnant. (c) Two years postoperatively, follow-up magnetic resonance angiogram (Volume Rendering Image) indicates that the aneurysmal dome has disappeared without remarkable regrowth
Clinical outcomes in the clip group
Within the first examined period, 79% of patients in the clip group showed positive clinical outcomes. Two patients (7.1%) experienced permanent AChA occlusion as a surgical complication. An additional 4 patients showed poor prognosis (mRS ≥ 3), including 2 with AChA syndrome because of AChA occlusion, 1 with cerebral contusion during surgery [
Surgery-related complications occurred in patients with types A, B1, C1, and C2 aneurysms. Three patients experienced incomplete clipping owing to wide neck type (B1) and type C (C1 and C2) aneurysms; however, the residual neck showed neither regrowth nor aneurysmal rupture at follow-up. The presence of AChA duplication appears to be the key issue inherent in surgical clipping. However, in this study, we could not preoperatively detect the AChA duplication in 3 cases (2 of type B2 and 1 of type C2), which were detected only during operation [Figures
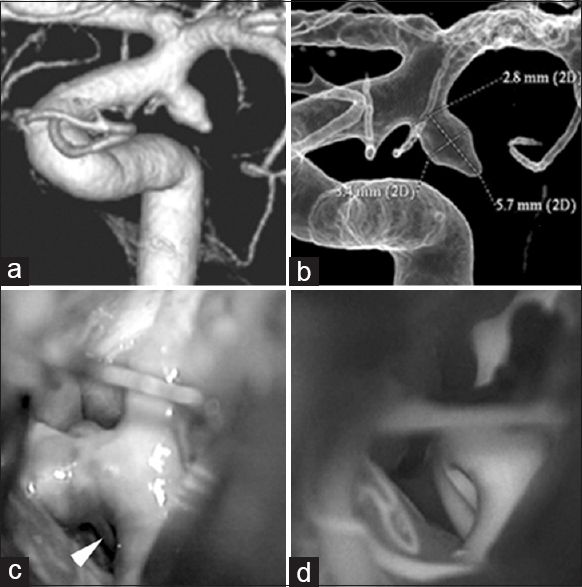
Figure 4
Findings in a 50-year-old man (a and b) Three-dimensional angiography demonstrates a single anterior choroidal artery (AChA) arising from the neck of the AChA aneurysm (classified as type B1). (c) Intraoperative photograph shows another branch of the AChA arising from the distal aneurysmal neck, undetected on preoperative angiography. This is classified as type B2 (white arrowhead). (d) Intraoperative indocyanine green-videoangiography demonstrates good flow without constriction of the AChA after surgical clipping
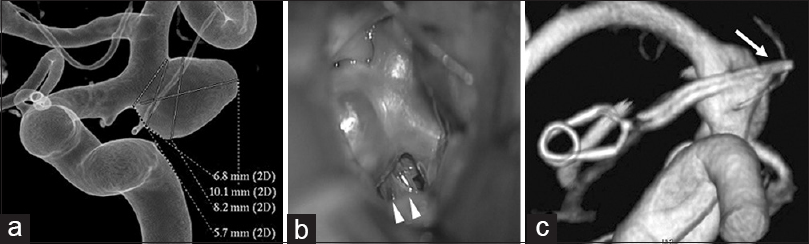
Figure 5
Findings in a 72-year-old woman (a) Preoperative 3-dimentional angiography demonstrates a single anterior choroidal artery (AChA) arising from the aneurysmal dome (classified as type C1). (b) Intraoperative photograph shows another branch of the AChA arising from the aneurysmal dome, undetected on preoperative angiography. This is classified as type C2 (white arrowhead). (c) Postoperative 3-dimensional angiography indicates preservation of the AChA, which is undetectable (white arrow)
DISCUSSION
The importance of anterior choroidal artery preservation
AChA aneurysms are rare, representing only 2–5% of all intracranial aneurysms, with few reports in the literature focusing on microneurosurgical treatment.[
Few reports have discussed surgical outcomes and aneurysmal classification based on the origin of the AChA. AChA aneurysms can be classified into 4 types based on their origin, namely, arising mainly from the internal carotid artery (artery type), arising from the aneurysmal neck (neck type), arising from the aneurysmal dome (dome type), and arising from the AChA itself (truncal type).[
Endovascular coiling
The recent developments in both the device and technique of endovascular treatment are remarkable. The double-catheter technique,[
Kang et al. analyzed complications after coil embolization and reported symptomatic complications occurring in the neck type (in this study, types B1 and B2).[
Surgical clipping
In cases of treatment with surgical clipping, some complications after AChA aneurysm have been previously reported. Cho et al. reported that the frequency of infarction was the highest in the neck type aneurysms, with a statistically significant difference.[
To preserve the AChA, type C aneurysms must be treated successfully with dome clipping (neck remnant) and not with coil treatment; these cases require long-term follow-up because of possible aneurysm regrowth, as observed in this study.[
Furthermore, in this study, we did not observe residual neck regrowth in the average follow-up period of 63.3 months.
Surgical clipping for AChA aneurysm has now become very safe—and the disadvantages inherent to endovascular coiling have virtually disappeared—owing to the advent of ICG-VAG and MEP monitoring. In our study, we found that the clinical outcomes of our patients in both the coil and clip groups were similarly favorable. Furthermore, we observed relatively good long-term outcomes in both groups. By carefully evaluating the aneurysmal type, the appropriate treatment method for each patient can be chosen. It appears that the best indications for coiling are type A aneurysm, a low dome-to-neck ratio, and high-grade SAH, whereas surgical clipping is safe for all types of aneurysms. In type B and C aneurysms, there is a slightly higher ratio of residual neck occurrence even after clipping. However, the long-term outcomes in our study population were good throughout the average follow-up period of 5.3 years.
CONCLUSION
In recent years, surgical clipping of AChA aneurysms has become safer because of the introduction of ICG-VAG and MEP monitoring. The selection of a treatment method—whether endovascular coiling or surgical clipping—should involve careful consideration of the relationship of the aneurysm with the neck and the AChA, as well as the presence of any AChA duplication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amagasaki K, Higa T, Takeuchi N, Kakizawa T, Shimizu T. Late recurrence of subarachnoid hemorrhage due to regrowth of aneurysm after neck clipping surgery. Neurol Med Chir. 2002. 42: 496-500
2. Baxter BW, Rosso D, Lownie SP. Double microcatheter technique for detachable coil treatment of large, wide-necked intracranial aneurysms. AJNR Am J Neuroradiol. 1998. 19: 1176-8
3. Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neuroform stent-assisted coil embolization of wide-neck intracranial aneurysms: Strategies in stent deployment and midterm follow-up. Neurosurgery. 2007. 61: 460-8
4. Bohnstedt BN, Kemp WJ, Li Y, Payner TD, Horner TG, Leipzig TJ. Surgical treatment of 127 anterior choroidal artery aneurysms: A cohort study of resultant ischemic complications. Neurosurgery. 2013. 73: 933-9
5. Cho MS, Kim MS, Chang CH, Kim SW, Kim SH, Choi BY. Analysis of clip-induced ischemic complication of anterior choroidal artery aneurysms. J Korean Neurosurg Soc. 2008. 43: 131-4
6. Drake CG, Vanderlinden RG, Amacher AL. Carotid-choroidal aneurysms. J Neurosurg. 1968. 29: 32-6
7. Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 2001. 94: 565-72
8. Galatius-Jensen F, Ringberg V. Anastomosis between the anterior choroidal artery and the posterior cerebral artery demonstrated by arteriography. Radiology. 1963. 81: 942-4
9. Kang HS, Kwon B, Kwon OK, Jung C, Kim JE, Oh CW. Endovascular coil embolization of anterior choroidal artery aneurysms. Clinical article. J Neurosurg. 2009. 111: 963-9
10. Kim BM, Kim DI, Chung EC, Kim SY, Shin YS, Park SI. Endovascular coil embolization for anterior choroidal artery aneurysms. Neuroradiology. 2008. 50: 251-7
11. Kim BM, Kim DI, Shin YS, Chung EC, Kim DJ, Suh SH. Clinical outcome and ischemic complication after treatment of anterior choroidal artery aneurysm: Comparison between surgical clipping and endovascular coiling. AJNR Am J Neuroradiol. 2008. 29: 286-90
12. Kodama Y, Ohnishi H, Taomoto K, Kuga Y, Nakashima K, Kubota H. The classification and surgery for anterior choroidal artery aneurysms based on morphological feature. Surg Cereb Stroke. 2011. 39: 267-71
13. Lasjaunias P, Berenctein A, Ter Brugge KG.editorsSurgical Neuroangiography 1, clinical vascular anatomy and variations. Heidelberg, Berlin: Springer-Verlag; 2001. p. 563-77
14. Lee YS, Park J. Anterior choroidal artery aneurysm surgery: Ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 2013. 54: 86-92
15. Lehecka M, Dashti R, Laakso A, van Popta JS, Romani R, Navratil O. Microneurosurgical management of anterior choroid artery aneurysms. World Neurosurg. 2010. 73: 486-99
16. Li J, Mukherjee R, Lan Z, Liu Y, He M. Microneurosurgical management of anterior choroidal artery aneurysms: A 16-year institutional experience of 102 patients. Neurol Res. 2012. 34: 272-80
17. Perria L, Viale GL, Rivano C. Further remarks on the surgical treatment of carotid-choroidal aneurysms. Acta Neurochir. 1971. 24: 253-62
18. Piotin M, Mounayer C, Spelle L, Williams MT, Moret J. Endovascular treatment of anterior choroidal artery aneurysms. AJNR Am J Neuroradiol. 2004. 25: 314-8
19. Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD. Prospective evaluation of surgical microscope–integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005. 103: 982-9
20. Shindou M, Acevedo JC, Turjman F. Aneurysmal remnants after microsurgical clipping: Classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir. 1998. 140: 1153-9
21. Sluzewski M, van Rooij WJ, Beute GN, Nijssen PC. Balloon-assisted coil embolization of intracranial aneurysms: Incidence, complications, and angiography results. J Neurosurg. 2006. 105: 396-9
22. Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J Neurosurg. 2006. 105: 675-81
23. Viale GL, Pau A. Carotid-choroidal aneurysms: Remarks on surgical treatment and outcome. Surg Neurol. 1979. 11: 141-5
24. Yasargil MG.editorsMicroneurosurgery. Stuttgart: Georg Thieme; 1984. II:
25. Yasargil MG, Yonas H, Gasser JC. Anterior choroidal artery aneurysms: Their anatomy and surgical significance. Surg Neurol. 1978. 9: 129-38