- Department of Surgery, Division of Neurosurgery, Chiang Mai University, Chiang Mai, Thailand.
Correspondence Address:
Chumpon Jetjumnong, Department of Surgery, Division of Neurosurgery, Chiang Mai University, Chiang Mai, Thailand.
DOI:10.25259/SNI_592_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kolakoth Pathoumthong, Chumpon Jetjumnong. Comparative study of subdural drain (SDD) versus sub periosteal drain (SPD) in treating patient with chronic subdural hematoma (CSDH). 24-Aug-2021;12:421
How to cite this URL: Kolakoth Pathoumthong, Chumpon Jetjumnong. Comparative study of subdural drain (SDD) versus sub periosteal drain (SPD) in treating patient with chronic subdural hematoma (CSDH). 24-Aug-2021;12:421. Available from: https://surgicalneurologyint.com/surgicalint-articles/11064/
Abstract
Background: Chronic subdural hematoma (CSDH) is common neurosurgical condition encountered in daily practice. Burr holes evacuation is standard treatment for symptomatic cases. Both subdural drain (SDD) and subperiosteal drain (SPD) have been reported to lower the recurrence rate when used in conjunction with burr holes. A randomized controlled trials were done to see if there were any differences in clinical and radiographic outcomes between the two types of drains.
Methods: A total of 42 CSDH patients were enrolled and allocated to one of two groups: SDD (n = 21) or SPD (n = 21). Demographic data, perioperative imaging characteristics, clinical outcome, and recurrence rate were recorded for comparison.
Results: In both groups, demographic characteristics such as sex ratio, mean age of patients, concomitant disease, and antithrombotic agent use were similar. At 6 months, 20 (95.2%) and 21 (100%) cases in the SDD and SPD groups, respectively, had a favorable outcome (mRS 0–3). Complete hematoma resolution at 6 months was achieved in 21 (100%) and 19 (90.5%) cases of the SDD and SPD groups, respectively. The amount of drain within 48 h was not difference between the two groups. None of the SDD recurred, but two of the SPD group did, necessitating reoperation, which had no effect on the final outcome.
Conclusion: These findings indicate that the drain type (SDD or SPD) has no effect on the outcome. The surgeon’s preference determines which procedure is used. Except in symptomatic circumstances, routine postoperative imaging may not be required.
Keywords: Burr hole, Chronic subdural hematoma, Drain
INTRODUCTION
Chronic subdural hematoma (CSDH) has been defined as liquefied hematoma in the subdural space with a characteristic outer membrane, predominantly hypodense or isodense crescentic collection along the cerebral convexity on cranial computed tomography (CT).[
MATERIALS AND METHODS
Study design and data analysis
This was a prospective randomized controlled trial study in CSDH patients treated by burr hole evacuation. Patients were randomly assigned to one of two groups (by a block of four randomization): patients with CSDH treated by burr hole evacuation with SDD placement or patients with CSDH treated by burr hole evacuation with SPD. The study protocol was approved by the Institutional Review Board (Study Code: SUR-2561-05431/Research ID: SUR-2561-05431). To compare primary and secondary outcomes, the Chi-square test and t-test were used in statistical analysis. Statistical significance was considered as P < 0.05.
Setting and selection criteria
This study included all adults over the age of 18 who were diagnosed with symptomatic CSDH between April 2019 and May 2020. The diagnosis was confirmed by CT scan or magnetic resonance imaging (MRI). We excluded nonsymptomatic patients and those who received conservative treatment; patients with CSDH due to a shunt over drainage; and all patients who had or required a craniotomy to remove CSDH, including those who were planned before surgery and those for whom we made a decision during surgery.
Data collection
We collect demographic data such as age, sex, duration of symptoms, Glasgow Coma Scale (GCS), modified Rankin score (mRS), concomitant diseases, anticoagulant/ antiplatelet use, hematoma thickness, midline shift (MLS), and hematoma appearance of CSDH on CT scan/MRI.
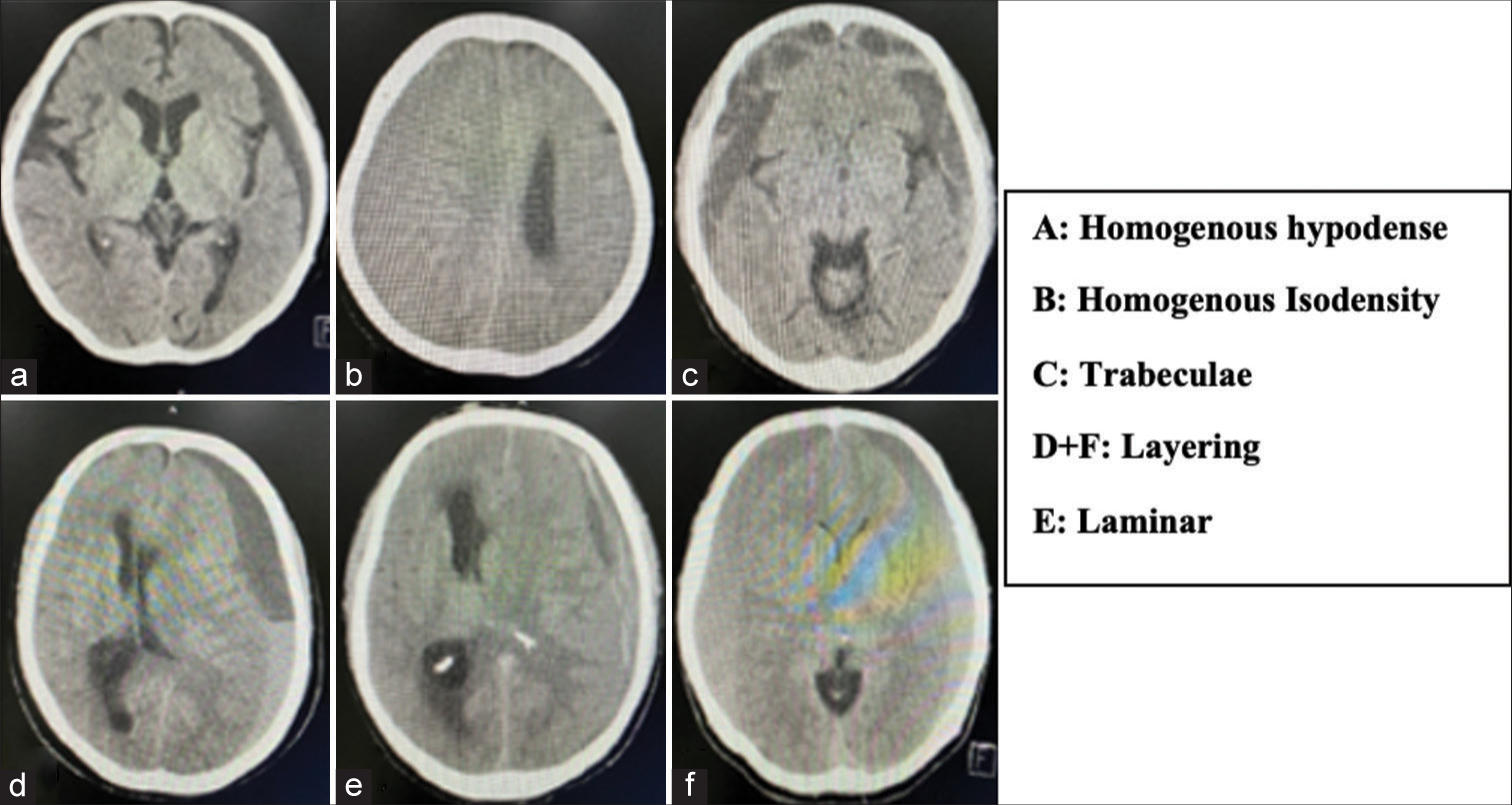
The appearance of a hematoma before and after surgery was divided into five types, including homogenous hypodense, homogenous isodensity, trabeculae, layering (separated), and laminar type [
Figure 1:
Chronic subdural hematoma classification based on hematoma appearance.[
The recurrence of CSDH was defined as initial clinical recovery followed by the development of CSDH-related symptoms with the radiographic appearance of hematoma. The primary outcome of this study was recurrence within 6 months, while the secondary outcomes were reoperation and complications (both intraoperative and postoperative). When the mRS was 0–3, the clinical outcome was considered favorable, and when the mRS was >4, it was considered unfavorable.
Operative procedure and perioperative care
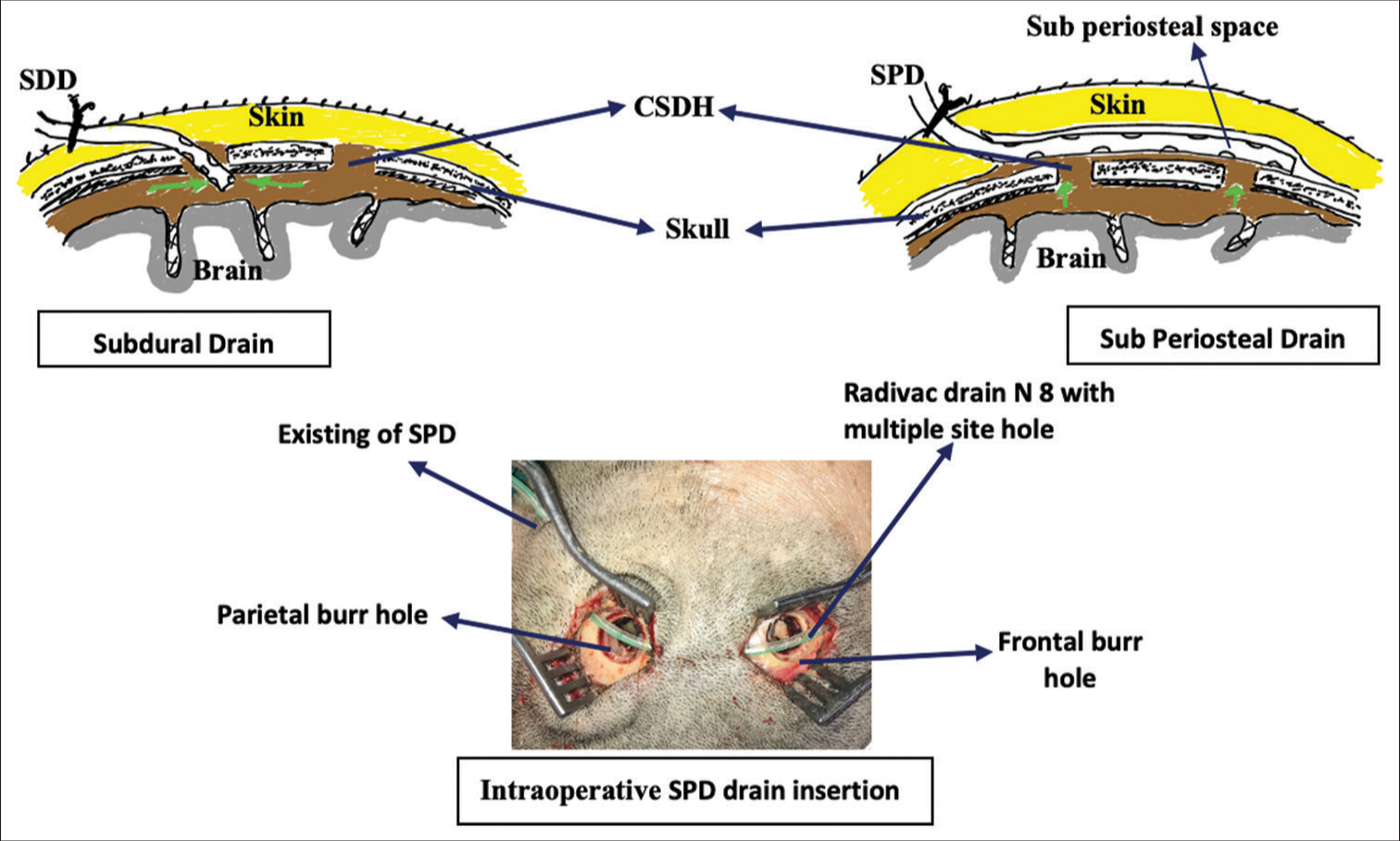
Before surgical evacuation, patients with coagulopathy (INR >1.4, platelet 100,000) or who had previously required antithrombotic or anticoagulant medication were treated. For surgical removal, all of the patients were put under general anesthesia. On the operation table, the patient was positioned supine on a doughnut headrest. Patients were administered a prophylactic antibiotic (single dosage) right before the skin incision and for 24 h afterward. Over the hematoma’s maximal thickness, two (13–15 mm) burr holes were drilled around 6–8 cm apart. The dura was opened in a cruciate fashion. The subdural collection was drained and the area was irrigated with warm saline until it was clear. Drain placement was done when hemostasis was obtained. For the SDD group, the nasogastric tube number 8 was inserted into the subdural space through a frontal burr hole <2 cm in depth and tunneled for 4–6 cm away from the frontal incision and the drain was connected to a ventriculostomy collection bag and was kept below the patient’s head level for gravity drainage. For the SPD group, a Redivac drain number 8 was placed subperiosteally and positioned to cover both burr holes, and it was connected to a suction drain with approximately 50% suction force applied [
RESULTS
Patient demographic data
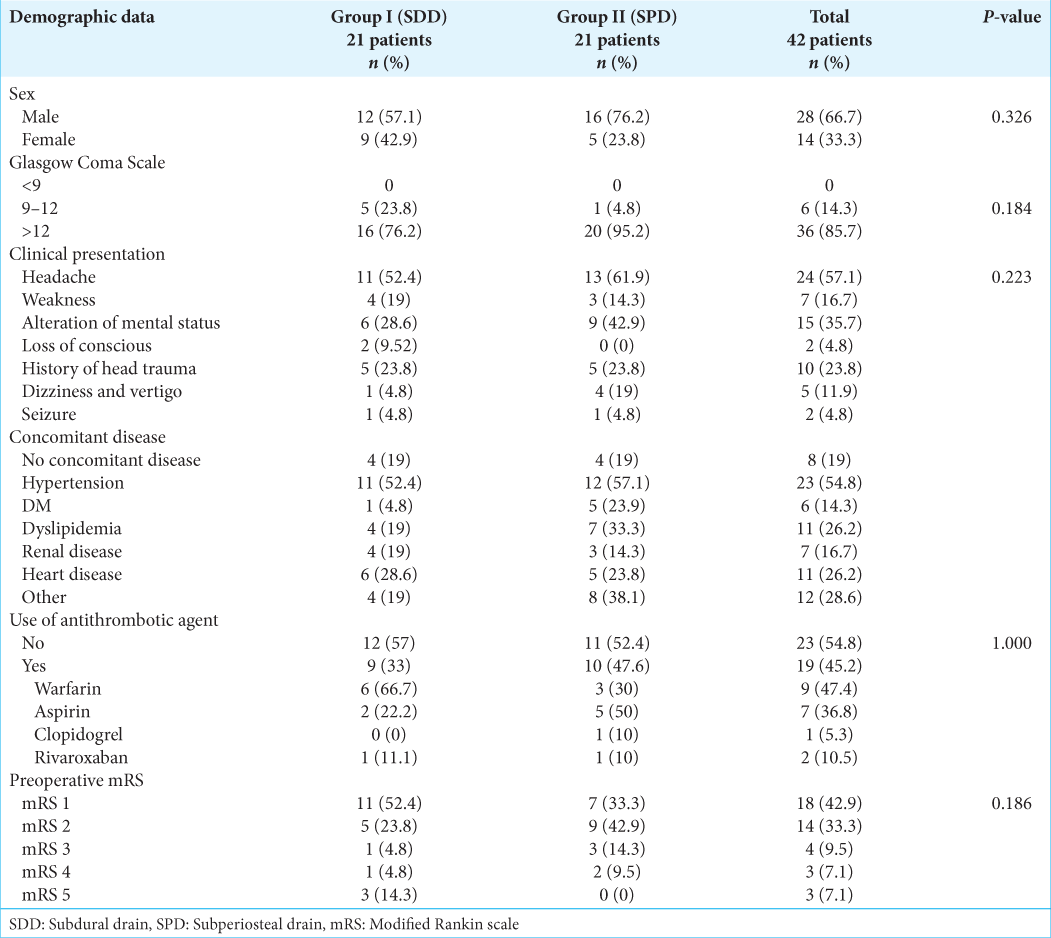
This study involved a total of 42 patients (n = 21 each group) and included 46 CSDH (4 of them had bilateral CSDH). In terms of demographic features, the two groups were comparable [
Perioperative imaging
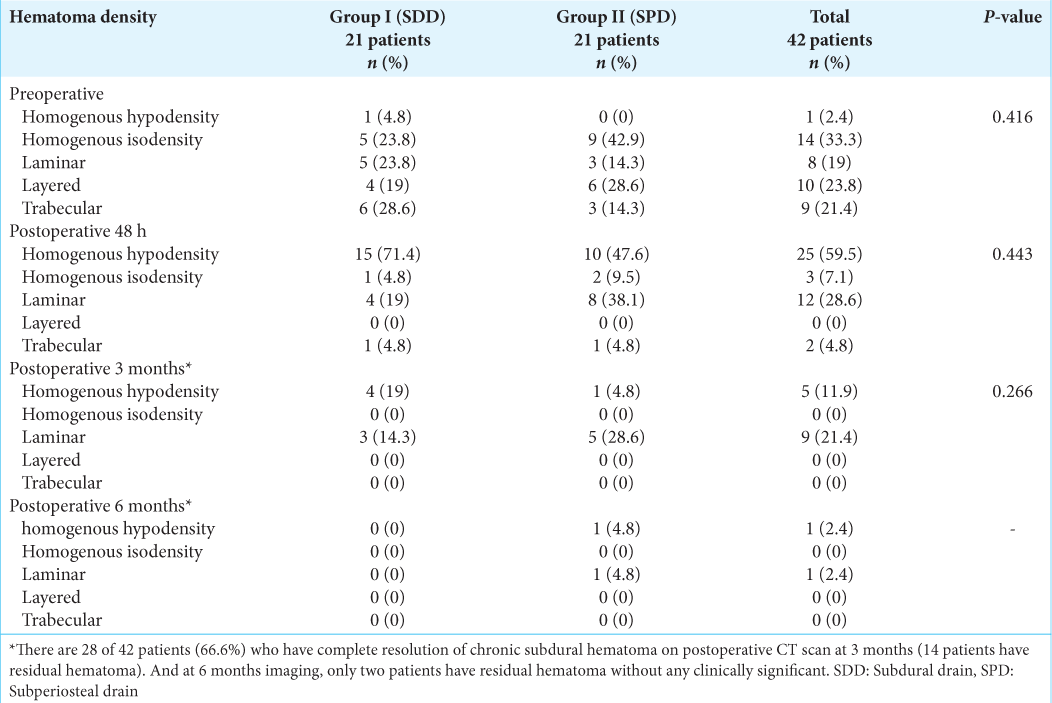
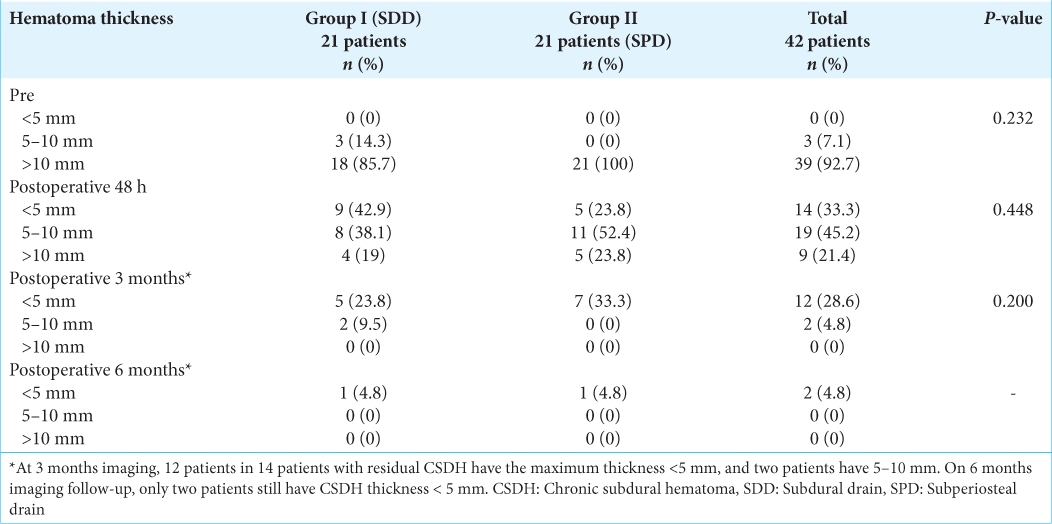
In both the preoperative and postoperative periods, imaging characteristics such as hematoma appearance, MLS, and thickness were comparable across the two groups. Preoperative hematoma appearance included homogenous hypodensity 2.4% (n = 1), homogenous isodensity 33.3 % (n = 14), laminar 19 % (n = 8), layered 23.8 % (n =10), and trabeculae type 21.4 % (n = 9) [
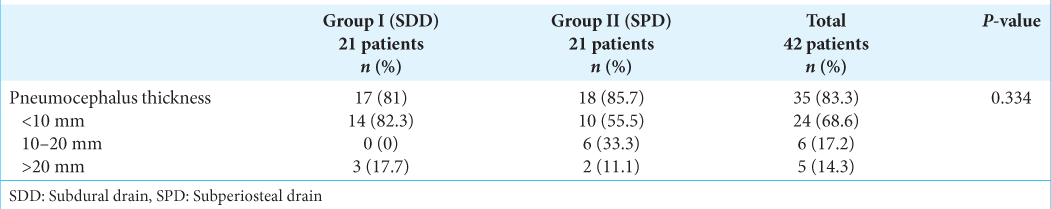
In both groups, the 48 h postoperative CT scan revealed improvement in hematoma density, MLS, and thickness. We found no statistically difference between the two groups (P = 0.443, P = 1.00, and P = 0.448, respectively). We noted that 83.3% (n = 35) of the patients had postoperative pneumocephalus but no clinical symptoms [
In most patients, the postoperative 3- and 6-month CT scans revealed interval resolution as expected. Only 4.8% (n = 2) of patients had a remnant hematoma < 5 mm thick at 6 months.
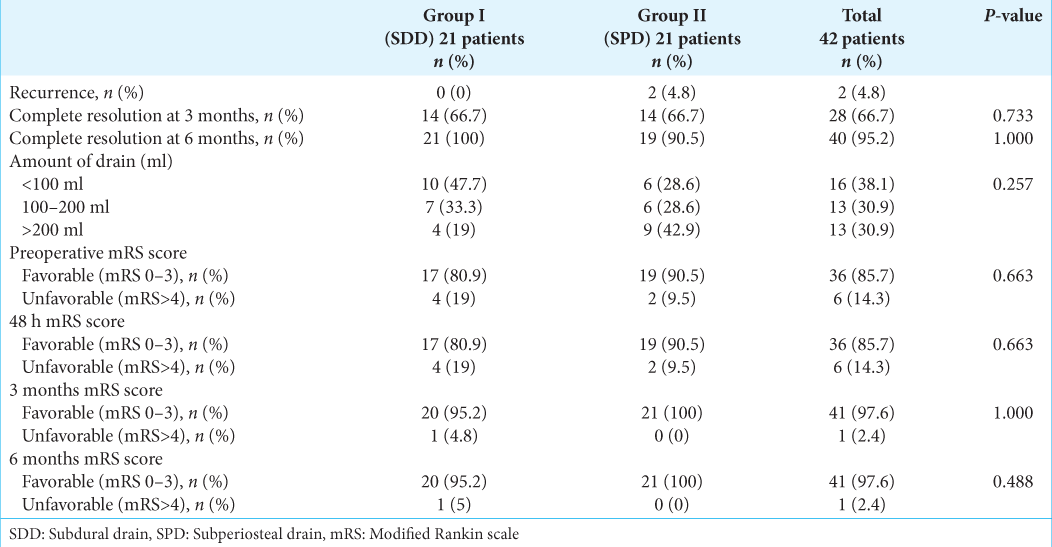
Clinical outcomes
In this study, we found two cases of recurrence and unfavorable outcome in the SPD group, but none in the SDD group. They needed to be reoperated within 48 h, but the final follow-up resulted in a favorable outcome. Between the SDD and SPD groups, there was no statistically significant difference in the rate of complete resolution at 3 and 6 months (66.7% vs. 66.7%, P = 0.733 and 100% vs. 90.5%, P = 1.000, respectively). The amount of drain appeared to be greater in the SPD group, but the difference was not statistically significant (P = 0.257). At 48 h, 3 months, and 6 months, the majority of patients had a positive result (mRS 0–3), with 85.7%, 97.6%, and 97.65%, respectively [
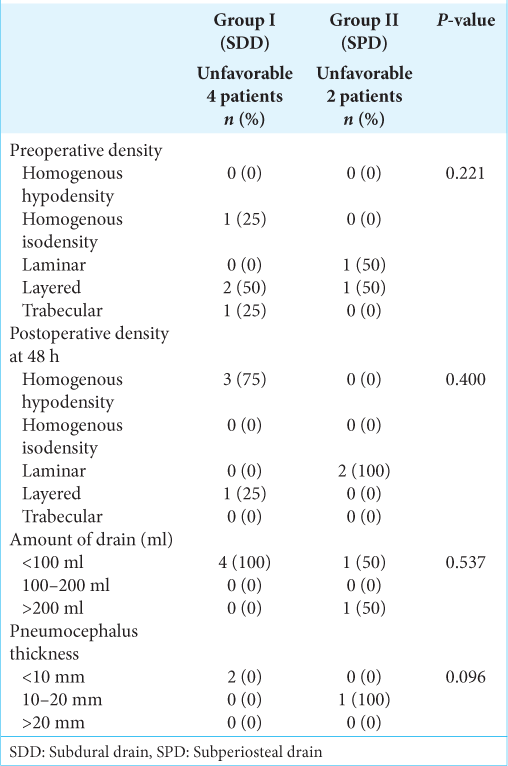
We looked at potential factors that could influence the unfavorable outcome, such as the appearance of CSDH (preoperative and 48 h postoperative), the amount of drain, and pneumocephalus, but none of them were statistically significant [
DISCUSSION
In neurosurgical practice, CSDH is a common condition. For symptomatic patients, surgical therapy is required. According to Level I evidence, burr hole evacuation with drain placement can greatly reduce the rate of recurrence.[
The current study found no statistically significant differences between SDD and SPD in terms of clinical outcomes (as defined by mRS), postoperative imaging characteristics, complication rate, or recurrence rate. Despite this, we found two cases of symptomatic recurrence in the SPD group that required reoperation within 48 h of surgery. In one case, the preoperative CT appearance was laminar, while in the other case, it was layered. We decided to reexamine the burr holes and we discovered and broke all remaining thin subdural membranes. SPD was placed after copious irrigation. Both of them have obtained favorable outcome (mRS 0) after 3 and 6 months of follow-up. We observed no clinical characteristics that could increase the likelihood of recurrence; nevertheless, other than the manner of drain insertion, the plausible explanation could be related to surgical skill or the surgeon’s experience.
Our findings were consistent with those of other studies. A nonrandomized prospective study by Chih et al. compared the efficacy of SDD versus SPD for the treatment of 30 symptomatic CSDH patients per group and found no significant statistical differences between the two groups in pre- and post-operative symptoms, postoperative hematoma volume and recurrence, mortality, or functional outcome at discharge and at the 3-month follow-up. In their study, they used a single burr hole technique and concluded that while both the SDD and SPD are equally effective, the SPD eliminates the risk of unintentional brain parenchymal penetration.[
All of our patients underwent follow-up imaging at 48 h, 3 months, and 6 months, which were an interesting aspect of our study. It is possible to examine the temporal profile of postoperative hematoma appearance. At 48 h, all patients had a remnant hematoma that was radiographically identified as homogeneous hypodensity (59.5%), homogenous isodensity (7.1%), laminar (28.6%), and trabecular type (4.8%). In 45.2% of cases, the MLS was resolved, 38.1% remained at 5 mm, and 16.7% remained at 5–10 mm. Our data suggest that postoperative residual hematoma and MLS that persist at 48 h do not necessitate reoperation and have no effect on the clinical outcome. Furthermore, because the majority of patients had complete radiographic remission after 3 months (66.6%) and 6 months (95.2%), we then propose that routine postoperative imaging is unnecessary, unless the patient remains symptomatic. In terms of amount of drain, the SPD group had considerably more patients with >200 ml drainage. (The SPD and SDD groups had nine and four patients, respectively.) This could be due to the suction effect of the Redivac suction drain in SPD versus the gravity drain in SDD. Many clinical and imaging factors that associated with recurrence have been reported in the literature, such as diabetic mellitus, preoperative headache, anticoagulant and preoperative MLS,[
The current study’s disadvantage is that we only had a limited sample size, therefore, the differences between the two groups were not significant. As a result, in the future, we advocate doing a prospective, randomized, multicenter study with a larger sample size to confirm our findings. In conclusion, the surgical technique for both types of drain placement is typically simple; most surgeons are more familiar with the SDD; but, for patients with limited subdural space following hematoma evacuation, the SPD may be the preferred option to avoid iatrogenic brain injury.
CONCLUSION
Our findings revealed that both SDD and SPD are equally effective in the treatment of CSDH patients, with no differences in final clinical and radiographic outcomes. The surgeon’s preference will determine which procedure is used. It is more crucial to have a good surgical technique. If the patient has no signs or symptoms of recurrence, postoperative imaging is not required. To reach a precise conclusion, a prospective, randomized, multicenter study with a larger sample size may be required.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: Results of a large single-center cohort study. Neurosurg Rev. 2004. 27: 263-6
2. Belkhair S, Pickett G. One versus double burr holes for treating chronic subdural hematoma meta-analysis. Can J Neurol Sci. 2013. 40: 56-60
3. Bellut D, Woernle CM, Burkhardt JK, Kockro RA, Bertalanffy H, Krayenbhl N. Subdural drainage versus subperiosteal drainage in burr-hole trepanation for symptomatic chronic subdural hematomas. World Neurosurg. 2012. 77: 111-8
4. Chih AN, Hieng AW, Rahman NA, Abdullah JM. Subperiosteal drainage versus subdural drainage in the management of chronic subdural hematoma (a comparative study). Malays J Med Sci. 2017. 24: 21-30
5. Fogelholm R, Heiskanen O, Waltimo O. Chronic subdural hematoma in adults, Influence of patient’s age on symptoms, signs, and thickness of hematoma. J Neurosurg. 1975. 42: 43-6
6. Gazzeri R, Galarza M, Neroni M, Canova A, Refice GM, Esposito S. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 2007. 149: 487-93
7. Ivamoto HS, Lemos HP, Atallah AN. Surgical treatments for chronic subdural hematomas: A comprehensive systematic review. World Neurosurg. 2016. 86: 399-418
8. Kaliaperumal C, Khalil A, Fenton E, Okafo U, Kaar G, O’Sullivan M. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 2012. 154: 2083-8
9. Kim SU, Lee DH, Kim YI, Yang SH, Sung JH, Cho CB. Predictive factors for recurrence after burr-hole craniostomy of chronic subdural hematoma. J Korean Neurosurg Soc. 2017. 60: 701-9
10. Kwon TH, Park YK, Lim DJ, Cho TH, Chung YG, Chung HS, Suh JK. Chronic subdural hematoma: Evaluation of the clinical significance of postoperative drainage volume. J Neurosurg. 2000. 93: 796-9
11. Lind CR, Lind CJ, Mee EW. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003. 99: 44-6
12. Macdonald RL.editors. Pathophysiology of chronic subdural hematomas. Youmans and Winn Neurological Surgery. Philadelphia, PA: Elsevier Inc; 2017. p. 5877-903
13. Motiei-Langroudi R, Stippler M, Shi S, Adeeb N, Gupta R, Griessenauer CJ. Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. J Neurosurg. 2018. 129: 1143-50
14. Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo). 2001. 41: 382-6
15. Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: A randomised controlled trial. Lancet. 2009. 374: 1067-73
16. Santarius T, Kolias AG, Hutchinson PJ.editors. Surgical management of chronic subdural haematoma in adults, Schmidek and Sweet Operative Neurosurgical Techniques: Indications. Methods and Results. Philadelphia, PA: Elsevier Inc; 2012. p. 1573-8
17. Smith MD, Kishikova L, Norris JM. Surgical management of chronic subdural haematoma: One hole or two?. Int J Surg. 2012. 10: 450-2
18. Soleman J, Kamenova M, Lutz K, Guzman R, Fandino J, Mariani L. Drain insertion in chronic subdural hematoma: An international survey of practice. World Neurosurg. 2017. 104: 528-36
19. Soleman J, Lutz K, Schaedelin S, Kamenova M, Guzman R, Mariani L. Subperiosteal vs subdural drain after burr-hole drainage of chronic subdural hematoma: A randomized clinical trial (cSDH-drain-trial). Neurosurgery. 2019. 85: E825-34
20. Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review. J Neurol Neurosurg Psychiatry. 2003. 74: 937-43
21. You CG, Zheng XS. Postoperative pneumocephalus increases the recurrence rate of chronic subdural hematoma. Clin Neurol Neurosurg. 2018. 166: 56-60
22. Zhang JJY, Wang S, Foo AS, Yang M, Quah BL, Sun IS. Outcomes of subdural versus subperiosteal drain after burr-hole evacuation of chronic subdural hematoma: A multicenter cohort study. World Neurosurg. 2019. 131: e392-401
23. Zumofen D, Regli L, Levivier M, Krayenbhl N. Chronic subdural hematomas treated by burr hole trepanation and a subperiostal drainage system. Neurosurgery. 2009. 64: 1116-21