- Departments of Anaesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Departments of Orthopedics, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Departments of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Hemant Bhagat
Departments of Anaesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_287_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Steve Joys, Tanvir Samra, Vishal Kumar, Manju Mohanty, Harsimrat B. S. Sodhi, Shalvi Mahajan, Hemant Bhagat. Comparison of postoperative delirium in patients anesthetized with isoflurane versus desflurane during spinal surgery: A prospective randomized controlled trial. 22-Nov-2019;10:226
How to cite this URL: Steve Joys, Tanvir Samra, Vishal Kumar, Manju Mohanty, Harsimrat B. S. Sodhi, Shalvi Mahajan, Hemant Bhagat. Comparison of postoperative delirium in patients anesthetized with isoflurane versus desflurane during spinal surgery: A prospective randomized controlled trial. 22-Nov-2019;10:226. Available from: http://surgicalneurologyint.com/surgicalint-articles/9770/
Abstract
Background: Following spine surgery, different types of inhalational anesthetic agents can result in postoperative delirium (POD) that can increase perioperative/postoperative morbidity. Here, we compared the incidence of POD in adults undergoing spine surgery anesthetized with isoflurane versus desflurane.
Methods: A prospective randomized double-blind clinical trial for patients undergoing spinal surgery was performed in 60 adults (aged 18–65 years); they were randomized to receive isoflurane or desflurane. On postoperative days 1 and 3, the diagnosis and severity of POD utilized 3D-Confusion Assessment Method (CAM) and CAM-severity delirium severity scores to assess patients’ status. Multiple other variables which may have influenced the frequency/severity of POD were also studied.
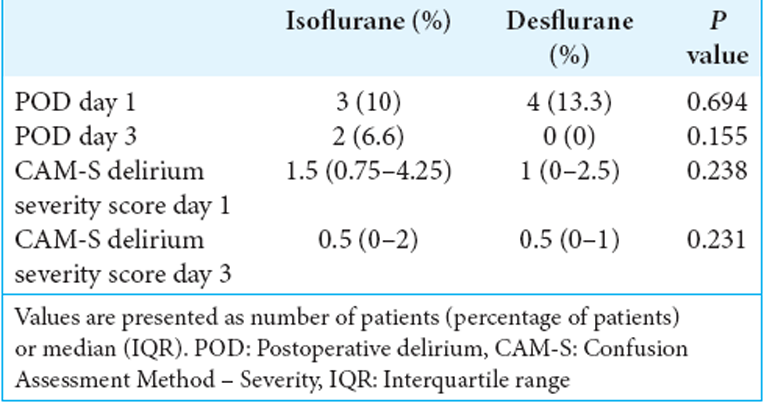
Results: For the two groups, the incidence of POD utilizing isoflurane and desflurane was similar on postoperative days 1 (10% vs. 13.3%, P > 0.05) and 3 (6.6% vs. 0%, P > 0.05). The severity scores of POD for both anesthetic agents were also similar on postoperative days 1 (1.5 vs. 1) and 3 (0.5 vs. 0.5). In addition, there was no significant association of POD with other perioperative factors.
Conclusion: A significant number of patients undergoing spine surgery experience POD. However, the incidence and severity of POD remained similar when utilizing either isoflurane or desflurane.
Keywords: Desflurane, Isoflurane, Postoperative delirium, Spine surgery
INTRODUCTION
Postoperative delirium (POD) occurs between 24 and 72 h after any surgery and can persist for months.[
Desflurane and isoflurane are two commonly used inhalational anesthetic agents. While animal studies have shown prolonged exposure to isoflurane may be neurotoxic, clinical series have reported faster emergence and less postoperative cognitive dysfunction (POCD) utilizing desflurane.[
MATERIALS AND METHODS
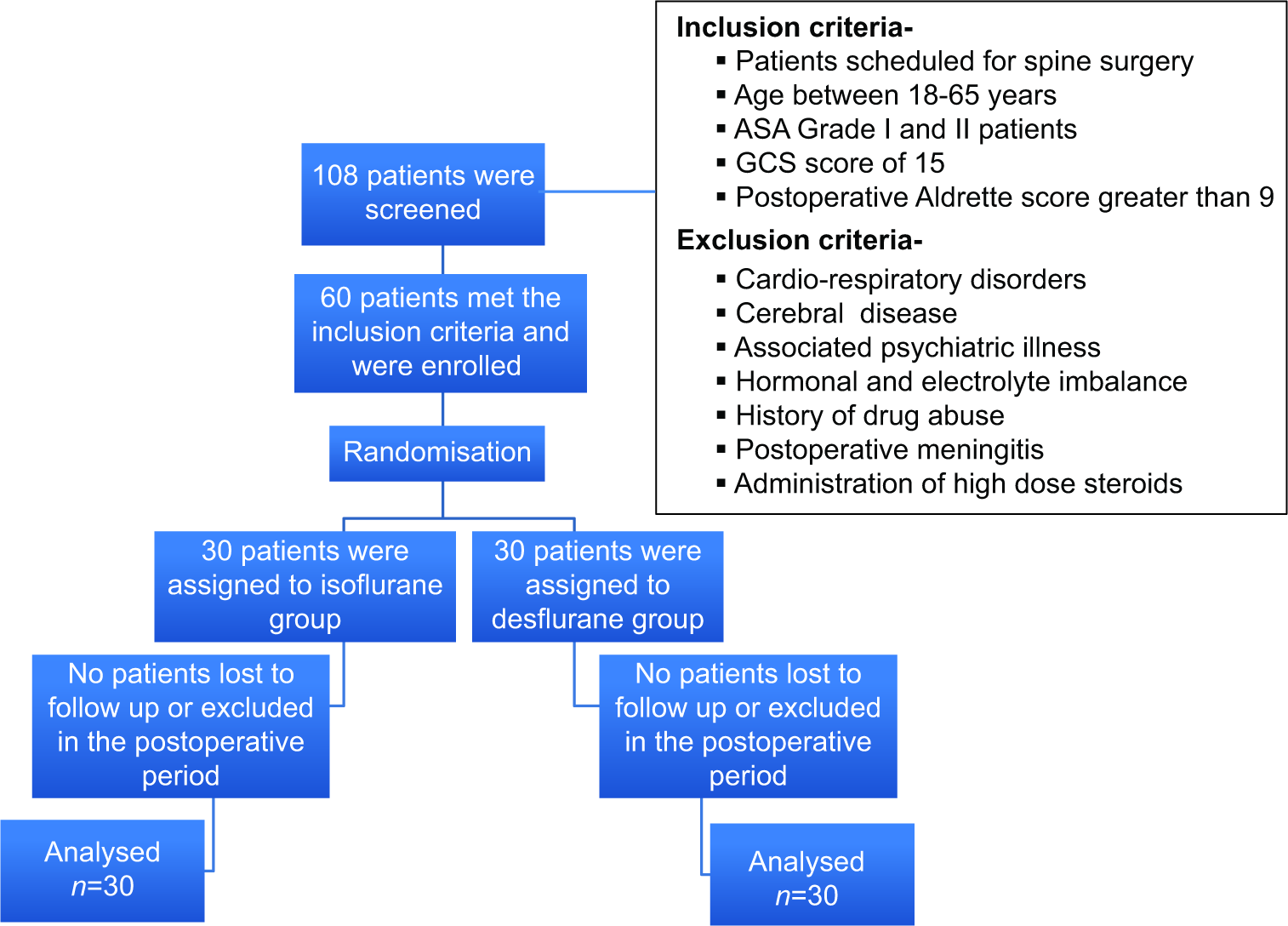
A prospective randomized double-blind clinical trial was conducted (2016–2017) in 60 patients, ages 18–65 years old, scheduled to undergo spine surgery; they provided written informed consent [
Anesthetic technique
Patients were screened for cognitive dysfunction preoperatively using the Mini-Cog Test.[
Postoperative assessment of POD
The diagnosis and severity of POD utilized the 3D-Confusion Assessment Method (CAM) and CAM-severity (CAM-S) long-form delirium severity score, respectively, on postoperative days 1 and 3.[
Statistical analysis
The results of a previously conducted study on POCD comparing isoflurane and desflurane were used to calculate the sample size in our study to prove the superiority of desflurane over isoflurane with a margin of at least 5% using an alpha error of 0.05 and power of 85%.[
RESULTS
POD results
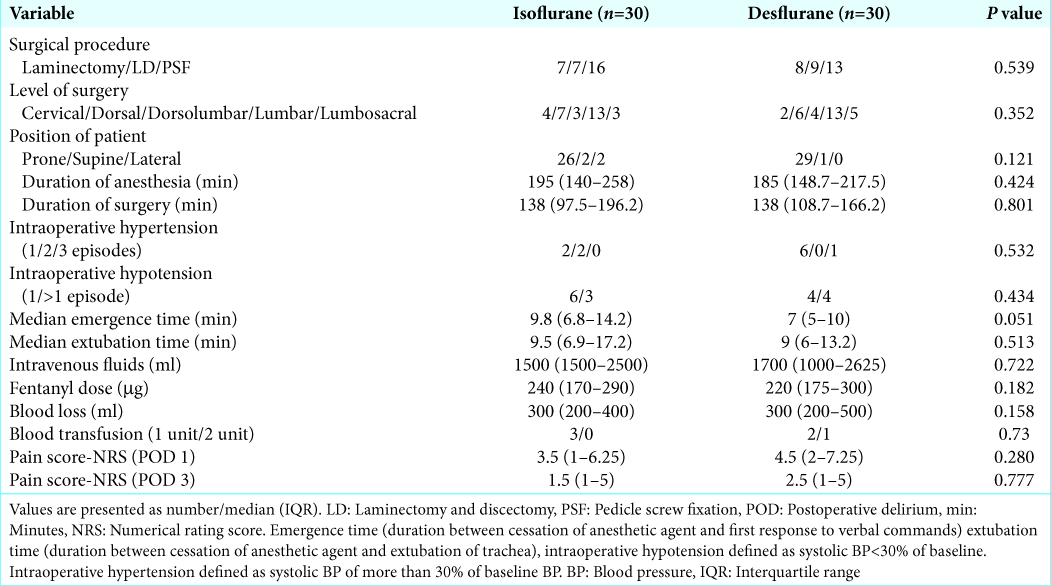
There were no statistically significant differences utilizing isoflurane versus desflurane on the incidence of POD between the two groups on postoperative days 1 (10% vs. 13.3%) and 3 (6.6% vs. 0%), respectively [
DISCUSSION
Comparable outcomes for POD with isoflurane versus desflurane
Our original hypothesis was that isoflurane may be associated with an increased incidence of POD versus desflurane in patients undergoing spinal surgery. We found the incidence and severity of POD in middle-aged adults undergoing spine surgery were similar on postoperative days 1 and 3.
Animal studies
Animal studies have demonstrated different incidences of neurotoxicity utilizing isoflurane and desflurane as inhalational anesthetics; some studies showed isoflurane to be neurotoxic, while others demonstrated both isoflurane and desflurane were neuroprotective.[
Prior incidence of POD in patients undergoing spine surgery
Previous studies on POD following spine surgery showed a higher incidence of delirium in patients older than 60 years.[
Limitations
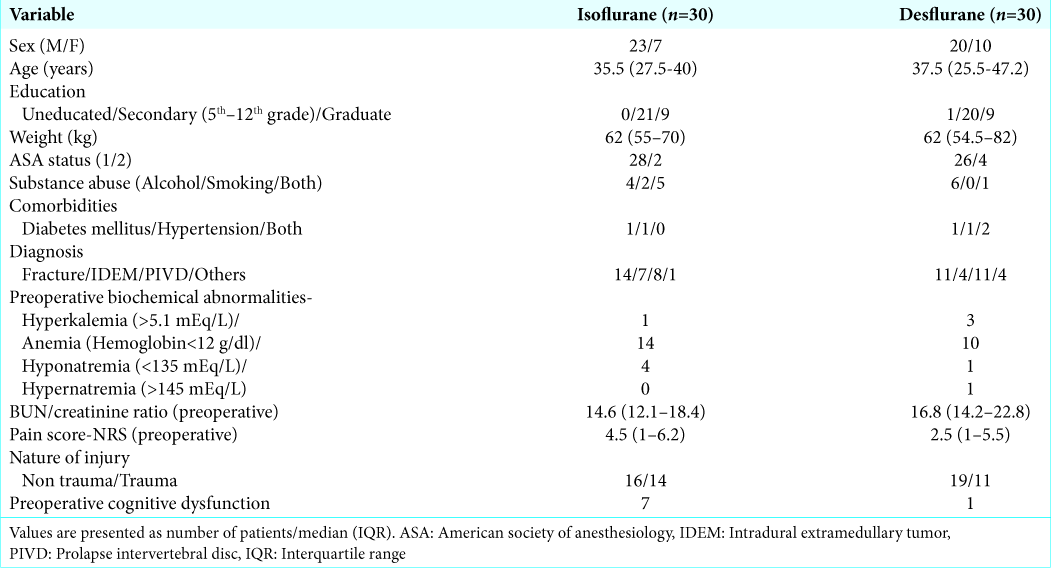
The study included only 60 patients randomized to two different anesthetic groups, and the study was only continued for 3 postoperative days. Further, the baseline incidence of preoperative cognitive dysfunction was higher in the isoflurane (n = 7) versus desflurane groups (n = 1).
CONCLUSION
The incidence and severity of POD in middle-aged patients receiving either isoflurane or desflurane for spine surgery were similar on postoperative days 1 and 3.
Ethics approval
Clearance to conduct the study was obtained from the Institute Ethics Committee on April 4, 2016 (reference number 10313/PG-2Trg/2015/5408-9), in accordance with the Helsinki Declaration of 1975, as revised in 2000. The trial was registered with clinicaltrials.gov on October 6, 2016 (clinicaltrials.gov identifier NCT02925611).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements and Disclaimer
Confusion Assessment Method. Copyright 2003, Hospital Elder Life Program, LLC. Not to be reproduced without permission.
No responsibility is assumed by the Hospital Elder Life Program, LLC for any injury and/or damage to persons or property arising out of the application of any of the content at hospitalelderlifeprogram.org.
References
1. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: A cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000. 15: 1021-7
2. Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010. 112: 834-41
3. Engelhard K, Werner C, Reeker W, Lu H, Möllenberg O, Mielke L. Desflurane and isoflurane improve neurological outcome after incomplete cerebral ischaemia in rats. Br J Anaesth. 1999. 83: 415-21
4. Gao R, Yang ZZ, Li M, Shi ZC, Fu Q. Probable risk factors for postoperative delirium in patients undergoing spinal surgery. Eur Spine J. 2008. 17: 1531-7
5. Grover S, Subodh BN, Avasthi A, Chakrabarti S, Kumar S, Sharan P. Prevalence and clinical profile of delirium: A study from a tertiary-care hospital in north India. Gen Hosp Psychiatry. 2009. 31: 25-9
6. Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS. The CAM-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014. 160: 526-33
7. Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011. 114: 578-87
8. Kawaguchi Y, Kanamori M, Ishihara H, Abe Y, Nobukiyo M, Sigeta T. Postoperative delirium in spine surgery. Spine J. 2006. 6: 164-9
9. Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K. Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology. 2011. 115: 979-91
10. Liu J, Wang P, Zhang X, Zhang W, Gu G. Effects of different concentration and duration time of isoflurane on acute and long-term neurocognitive function of young adult C57BL/6 mouse. Int J Clin Exp Pathol. 2014. 7: 5828-36
11. Loepke AW, Istaphanous GK, McAuliffe JJ, Miles L, Hughes EA, McCann JC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009. 108: 90-104
12. Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008. 106: 1681-707
13. Marcantonio ER, Ngo LH, O’Connor M, Jones RN, Crane PK, Metzger ED. 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: A cross-sectional diagnostic test study. Ann Intern Med. 2014. 161: 554-61
14. Pensado Castiñeiras A, Rama Maceiras P, Molins Gauna N, Fiqueira Moure A, Vásquez Fidalgo A. Immediate anesthesia recovery and psychomotor function of patient after prolonged anesthesia with desflurane, sevoflurane or isoflurane. Rev Esp Anestesiol Reanim. 2000. 47: 386-92
15. Silverstein JH, Timberger M, Reich DL, Uysal S. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology. 2007. 106: 622-8
16. Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011. 77: 448-56
17. Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012. 114: 410-5