- Department of Neurosurgery, Azienda Ospedaliero Universitaria di Sassari, Via Enrico De Nicola, Sassari, Italy.
DOI:10.25259/SNI_35_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Domenico Policicchio, Gina Casu, Giosuè Dipellegrini, Artan Doda, Giampiero Muggianu, Riccardo Boccaletti. Comparison of two different titanium cranioplasty methods: Custom-made titanium prostheses versus precurved titanium mesh. 13-Jun-2020;11:148
How to cite this URL: Domenico Policicchio, Gina Casu, Giosuè Dipellegrini, Artan Doda, Giampiero Muggianu, Riccardo Boccaletti. Comparison of two different titanium cranioplasty methods: Custom-made titanium prostheses versus precurved titanium mesh. 13-Jun-2020;11:148. Available from: https://surgicalneurologyint.com/surgicalint-articles/10077/
Abstract
Background: The aim of this study was to compare the results of two different titanium cranioplasties for reconstructing skull defects: standard precurved mesh versus custom-made prostheses.
Methods: Retrospective analysis of 23 patients submitted to titanium cranioplasty between January 2014 and January 2019. Ten patients underwent delayed cranioplasty using custom-made prostheses; and 13 patients were treated using precurved titanium mesh (ten delayed cranioplasties, and three single-stage resection- reconstructions). Demographic, clinical, and radiological data were recorded. Results and complications of the two methods were compared, including duration of surgery, cosmetic results (visual analog scale for cosmesis [VASC]), and costs of the implants.
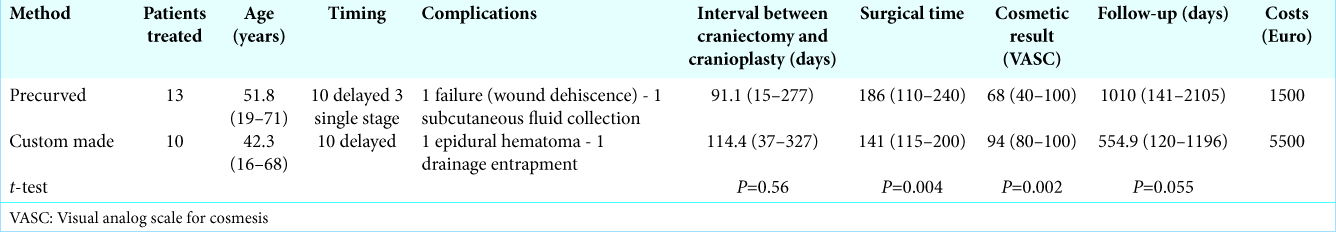
Results: Complications: one epidural hematoma in the custom-made group, one delayed failure in precurved group due to wound dehiscence with mesh exposure. There were no infections in either group. All custom-made prostheses perfectly fitted on the defect; eight of 13 precurved mesh prostheses incompletely covered the defect. Custom-made cranioplasty obtained better cosmetic results (average VASC 94 vs. 68), shorter surgical time (141min vs. 186min), and -fewer screws was needed to fix the prostheses in place (6 vs. 15). However, satisfactory results were obtained using precurved mesh in cases of small defects and in single-stage reconstruction. Precurved mesh was found to be cheaper (€1,500 vs. €5,500).
Conclusion: Custom-made cranioplasty obtained better results and we would suggest that this should be a first choice, particularly for young patients with a large cranial defect. Precurved mesh was cheaper and useful for single-stage resection-reconstruction. Depending on the individual conditions, both prostheses have their place in cranioplasty therapies.
Keywords: Cranioplasty, Custom-made cranioplasty, Decompressive craniectomy, Precurved mesh, Titanium mesh
INTRODUCTION
The management of patients with cranial defects (craniolacunias) has become a very common problem for neurosurgeons over the years. The use of decompressive craniectomy for the emergency treatment of several pathologic conditions such as severe head injuries, ischemic, and hemorrhagic strokes, cerebral venous thrombosis, severe brain infections, and subarachnoid hemorrhages,[
Clinical experience and literature data show that cranioplasty, despite seeming a technically simple procedure, has an average complication rate of 10–20% with a risk of failure (through infection or graft resorption) of about 10%.[
Reconstruction of the head defect has two main purposes: to guarantee protection for the brain and to restore adequate cosmetics; the results must be long lasting. The main complications of cranioplasty are represented, in addition to the investigated aesthetic result, by risk of infection, postoperative hematoma, problems with wound healing and even long-term failure by graft resorption or infections with consequent need for removal of the prostheses.
Theoretically, the ideal material for cranial reconstruction is autologous bone flap since it does not present biocompatibility issues, its shape is perfect for restoring normal cosmetics, and it ensures immediate and adequate protection for intracranial structures. Regardless of the difficulties in adequately storing the bone flap, however, it has been found (in several literature reports) that even autologous bone has rather high failure rates related to infection or resorption.[
Titanium cranioplasty can be performed in two ways, using precurved meshes (which are then adapted to the individual patient) or using custom-made prostheses that are specifically reconstructed for the individual patient using computer-aided design/computer-aided manufacturing techniques. The two prostheses differ in terms of costs and results. Within the literature, there are numerous reports about titanium cranioplasty with custom-made prostheses, although relatively few articles discuss the use of precurved meshes. We have, therefore, compared these two types of prostheses.
The aim of this study was to compare the results of two different titanium cranioplasties in reconstructing skull defects following craniectomy: standard precurved mesh versus custom-made prosthesis; the final aim was to assess whether one of the two prostheses should be excluded, or whether both can be useful in different situations.
MATERIALS AND METHODS
Study design
All patients enrolled in the study provided their written consent for anonymous data collection and inclusion in the study. We carried out a retrospective analysis of the patients who underwent cranioplasty surgery at our center in the period between January 2014 and January 2019. We used four types of cranioplasty during this period: cryostored autologous bone flap, manually shaped PMMA cranioplasty, custom-made HA cranioplasty, custom-made titanium cranioplasty, and precurved titanium mesh.
For this study, we selected and included patients undergoing titanium cranioplasty; we reviewed the medical records, radiological data (pre- and postoperative), and operating reports; we noted the demographic data, age at the time of surgery, cause of head defect, time elapsed between defect acquisition and reconstruction, any risk factors for cranioplasty failure, and duration of cranioplasty surgery and any complications (early and late); we finally requested that patients (or family members for patients with persistent neurocognitive deficits) completed a questionnaire covering subjectively-experienced satisfaction with the cosmetic result based on 100-mm-long visual analog scale (VASs) (VAS for cosmesis [VASC]).[
Statistical analysis was performed using the t-test. P < 0.05 was considered statistically significant.
Two types of prostheses were used: precurved and custom made. During the study period, the two prostheses were not always available in our department, and the choice of which prostheses to use was partly dependent on the hospital company being able to purchase one or the other.
Description of the two prostheses
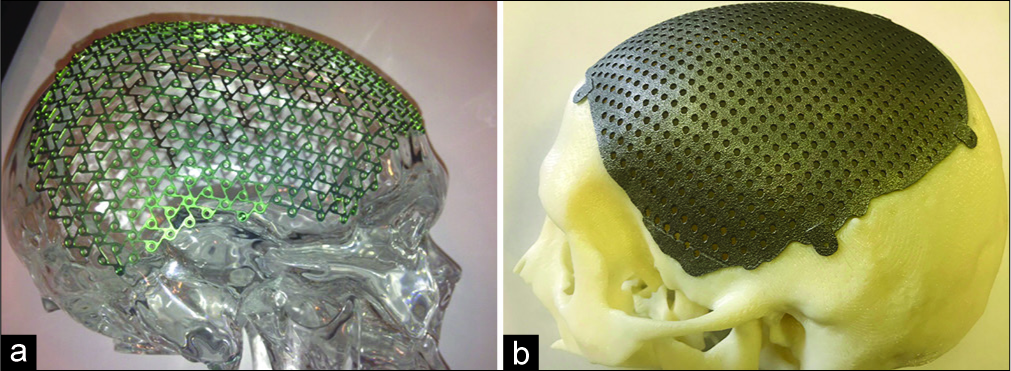
CranioCurve Preformed Mesh (Zimmer Biomet) [example in
Custom-made prostheses (MT Ortho) [example in
Surgical procedure
The operation was performed under general anesthesia in the supine position. A first dose of a broad-spectrum antibiotic agent was given intravenously at induction of anesthesia, then one dose every 4 h for the next 24 h. The patient’s hair was completely removed using hair clippers. Great care was taken to avoid skin damage. Skin was thoroughly washed and then disinfected. An iodine- impregnated incision drape was placed over the exposed skin, and care was taken to ensure that all surfaces were covered. In three cases, where cranioplasty was performed at the same time as a tumor removal operation involving the cranial bone (patient number 21, 22, and 23, single- step resection-reconstruction), we used the neuronavigator to plan skin flap and craniectomy and iUS to guide tumor resection (according to our protocol described elsewhere);[
RESULTS
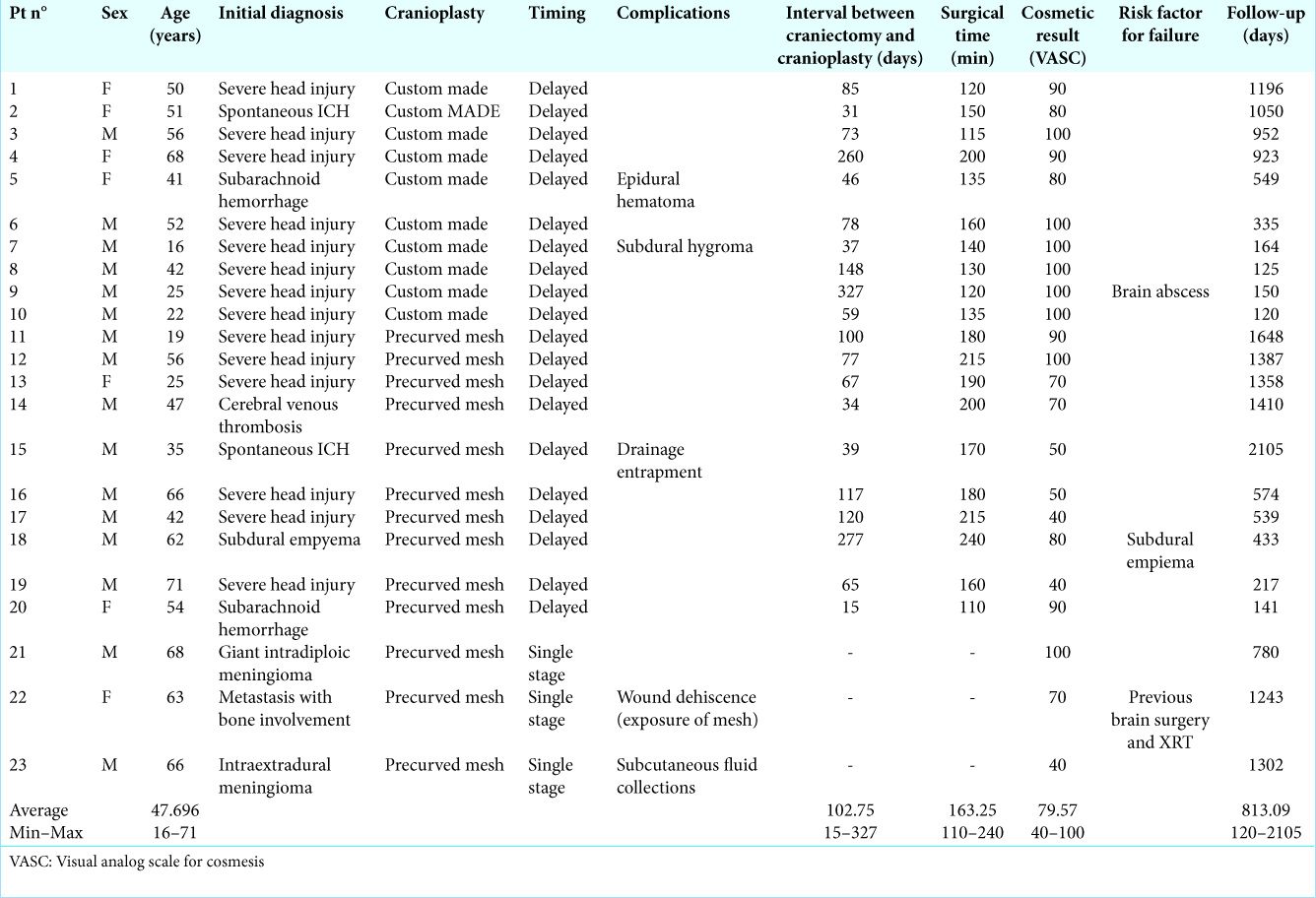
From January 2014 to January 2019, 23 patients underwent cranioplasty using titanium prostheses. Average age 47.6 years (minimum 16 years, maximum 71 years); 16 males and 7 females. Average follow-up time 813 days (minimum 120 days, maximum 2105 days), [
Three patients underwent tumor removal surgery with intradiploic extension (with removal of the involved bone) and immediate reconstruction using precurved titanium mesh (single-stage). The remaining 20 were submitted to delayed cranioplasty after decompressive craniectomy for severe head trauma (14 patients), intraparenchymal hemorrhage (two patients), subarachnoid hemorrhage (two patients), cerebral venous thrombosis (one patient), and subdural empyema (one patient). The mean time interval between craniectomy and cranioplasty was 89 days (minimum 15 days, and maximum 327 days). Twenty patients had cranial defect in the frontotemporo-parietal region, one frontotemporal defect, one frontotemporal- orbital defect, and one biparietal defect at the vertex.
Precurved titanium meshes were used in 13 patients (ten patients undergoing decompressive craniectomy and three undergoing single-step resection-reconstruction); and custom-made titanium cranioplasty was used in ten patients. [
In one patient who underwent decompressive craniectomy for subdural empyema with encephalitis, custom-made mesh was prepared based on a 3D CT scan performed 45 days after surgery. Due to systemic problems related to infectious disease, clinical conditions stabilized very slowly, and cranioplasty was performed after 277 days. Intraoperatively, custom-made prostheses resulted inadequate because of bone defect enlargement (probably due to the progression of infectious disease with osteomyelitis) and therefore the surgery was performed using a precurved mesh. The subsequent postoperative course was uneventful and without complications. This patient was included in the precurved mesh group. In all patient in the custom made group we observed a perfect fit of the mesh on the head defect, this clinical observation was also confirmed by the revision of postoperative imaging [
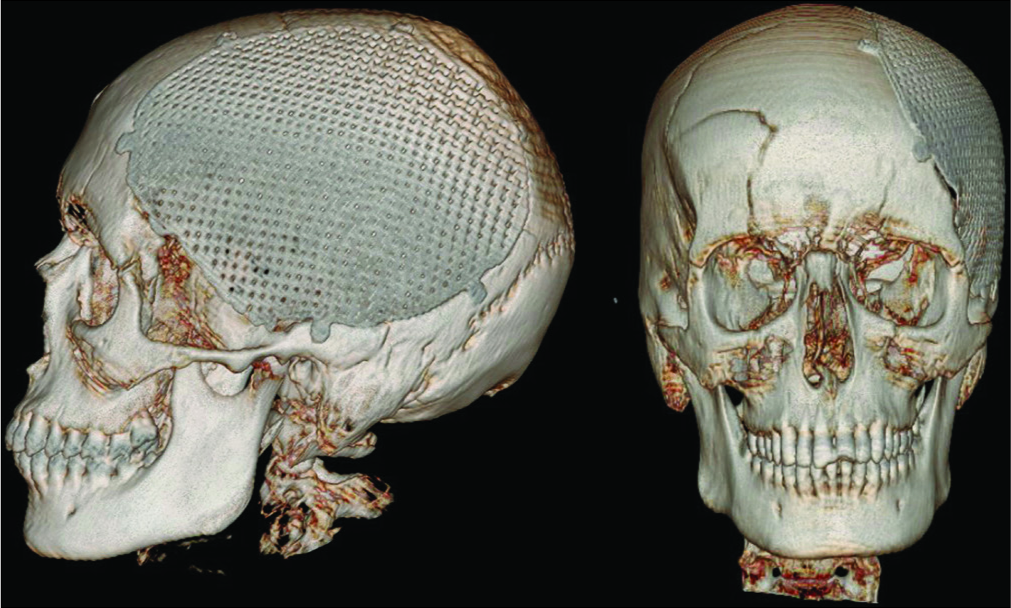
Figure 2:
Postoperative 3D computed tomography reconstruction of a patient treated using custom-made titanium cranioplasty; the implant completely cover cranial defect and obtain adequate cosmetic result, shape and curvature appear symmetric to contralateral side (patient seven, and visual analog scale for cosmesis 100).
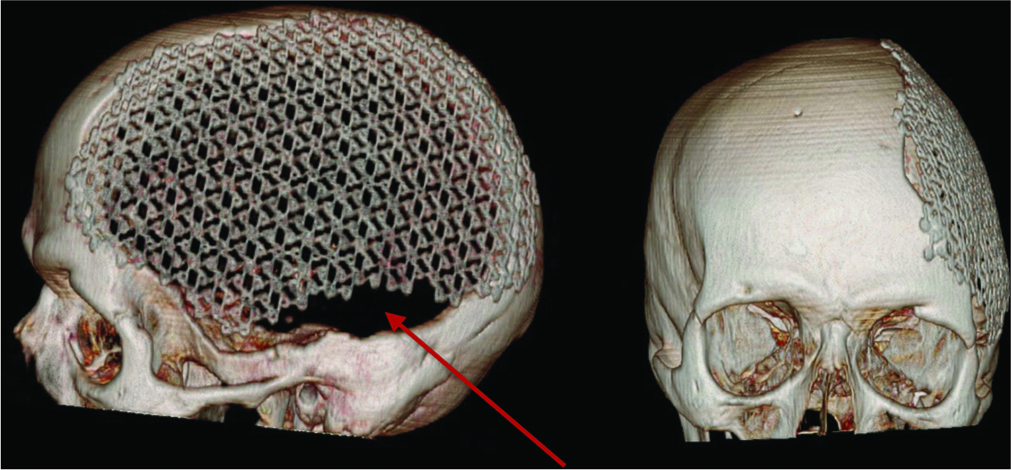
In eight of the 13 patients in whom precurved mesh was used there was no adequate fitting of the mesh on the head defect with persistence of areas not covered by the prostheses [
Figure 3:
Postoperative 3D computed tomography reconstruction of a patient treated using precurved titanium mesh; the cranial defect is not completely covered (red arrow) with persistent small defect in the anterior and inferior portion of the initial defect. Cosmetic result is not satisfactory (patient eight, and visual analog scale for cosmesis 40).
Duration of operation: average surgical time of precurved mesh was 186 min (minmum 110–maximum 240). Average for custom-made prostheses was 141 min (minimum 115– maximum 200); average difference of 45 min with a value of P = 0.04. The three patients who underwent immediate reconstruction after tumor removal were not counted in this evaluation.
Early complications: precurved group: one wound drainage incarceration (removed in the operating room under local anesthesia); and one subcutaneous seroma conservatively treated. Custom-made group: one epidural hematoma surgically evacuated; and one subdural hygroma conservatively treated. Late complications one skin dehiscence, with underlying precurved mesh exposure in patient with multiple cranial surgeries and radiotherapy; dehiscence occurred at a point where the mesh adequately adhered to the cranial defect, no tilt or lump was present; and indication was given for removal of the prostheses but the patient declined. We had no infections. No prostheses were removed. Cosmetic results: precurved VASC average 68 (40– 100), custom-made 94 (80–100); test t P = 0.002.
DISCUSSION
The main objectives of cranioplasty are cosmetics and the protection of the brain. Although there is a great deal of evidence to suggest that cranioplasty can also have a positive effect on neurological outcome (“Syndrome of the Trephined”),[
Evaluating the major complications, we obtained similar results in the two groups with a major complication for each type of prostheses: a failure (skin dehiscence with prostheses exposure) for the precurved, and an epidural hematoma surgically evacuated for the custom-made group. We had no infections.
Analyzing the single complications, the absence of infections in this series of patients confirmed the literature data showing the low infection rate of titanium compared to other materials.[
We obtained only one epidural hematoma in a patient with custom-made prostheses who required surgical evacuation. In the whole group, therefore, we had an incidence of 4.3%, which is in line with the data in the literature that report incidences between 2% and 5%,[
We only had one case of failure due to skin dehiscence with exposure of the underlying precurved mesh; although the patient denied to have the prostheses removed due to personal problems, this case must be counted as a failure because the exposed prostheses created a high risk of infection and could lead to the progression of skin dehiscence. The patient had multiple previous surgeries and radiotherapy for skull-base metastases, so we believe that this complication is related to the characteristics of the patient rather than to kind of prostheses. This observation is based on the fact that previous studies have identified several risk factors that contribute toward a poor outcome and failure in cranioplasty, such as previous irradiation, multiple previous operations, communication with the paranasal sinuses, inadequate delay between craniectomy and cranioplasty, and previous infection.[
The main differences between the two prostheses were the duration of the surgery, esthetic result, cost, and time required for production. We did not assessed the postoperative time to discharge because (in our series) it appeared to be mainly dependent on the characteristics of the patient rather than on the type of prostheses used.
The duration of the surgery was on average shorter by around 45 min in the custom-made group [
The cosmetic result was assessed using a VASC scale, the score was assigned by the patient (or their family members), but in all cases, the VASC score was consistent with clinicians’ evaluations. Custom-made cranioplasty obtained a higher average score [94 vs. 68, Table 2], with a value of P = 0.002. This result was predictable given the technical difficulties of adequately reproducing the normal shape and cranial conformation; the data were widely discussed in the literature, in which many authors showed the superiority of the cosmetic result of custom-made prostheses, both in titanium[
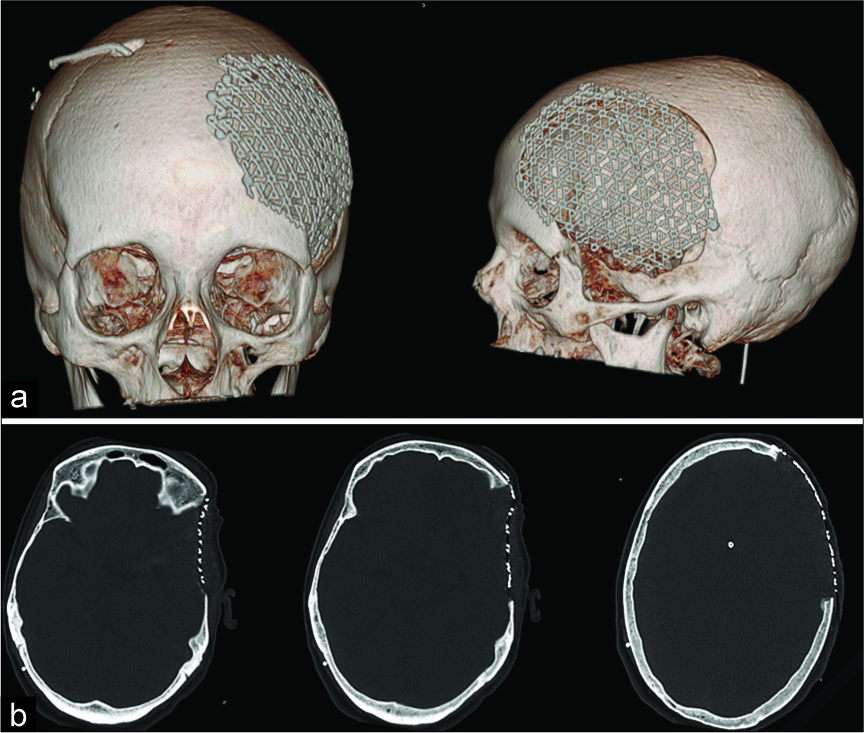
Figure 4:
Postoperative computed tomography scan of a patient treated using precurved titanium mesh: (a) 3D reconstruction, (b) three different axial slices of the same exam. The defect is relatively small, although the defect is not completely covered by the mesh, cosmetic result is satisfactory (patient 20, and visual analog scale for cosmesis 90).
The cost of precurved meshes is 1500 € and that of custom- made meshes is about 5500 €; the difference is partly absorbed by the costs of the screws, which are much more numerous in precurved meshes. The high number of screws for this type of prostheses is required to facilitate adhesion with the bone edges and to reduce the risk of tilt under the skin. These costs appear to be in line with what has been reported by other authors. All custom-made cranioplasties have similar costs with differences related to the size of the defect and the material used: PMMA appears to be the least expensive (but with the highest rate of infection), with titanium and PEEK being the most expensive.[
The last difference between the two groups in our current study was the time taken to obtain the prostheses. The precurved meshes, after purchase, were always available in the hospital for implantation even in emergency conditions; the production time for custom-made prostheses (from the execution of the CT to the actual availability in the hospital) was 20–30 days. This time delay is irrelevant in patients who had to undergo delayed cranioplasty after decompressive craniotomy; these patients (who represent the majority in almost all series), required a reasonable period of time before the reconstruction of the cranial defect to promote the stabilization of clinical conditions and the regression of the cerebral swelling. As such, cranioplasty surgery was usually planned in advance, and consequently the 3–4 weeks necessary for obtaining the prostheses caused no problems. There are cases, however, in which it may be useful to reduce the wait for cranioplasty. This would be particularly useful for cases of single stage surgery (resection and reconstruction): neoplasms with bone involvement, head trauma with comminute fracture, and also infectious diseases with bone involvement as proposed by Ehrlich et al. The authors performed immediate titanium cranioplasty for treatment of postcraniotomy infection (manually shaped mesh). In their series (24 patients), only two patients were reoperated (but not due to prostheses infection), and all patients who completed the evaluation questionnaire (20/24) said they were satisfied with the esthetic result.[
Study limitations
Our study had limitations, since the study was retrospective and the sample size was rather small. The two groups have similar demographic and etiopathological features, but the small sample number prevented the extrapolation of any definitive conclusions. The results must be integrated with data from literature and on a broader array of cases, particularly for appropriate and complete economic and health observations.
CONCLUSION
In our study, we carried out a retrospective evaluation of two types of titanium cranioplasty: precurved meshes compared with custom-made prostheses. The two groups of patients had similar demographic and clinical characteristics. We evaluated the differences of the two methods, rates of complications, esthetic results, surgical times, and material costs.
In the final comparison, the two prostheses showed overlapping rates of complications in line with the data of the literature; custom-made cranioplasties had a much better aesthetic result, perfect and complete coverage of the head defect, shorter surgical times, and involved an easier operation for the surgeon. Precurved meshes were cheaper, with satisfactory esthetic results in small defects, and were always available even for emergencies or for immediate reconstruction; in case of large defects, the cosmetic result is, on average, lower, with an increased surgical time and incomplete coverage of the craniolacunia in a high percentage of patients. Therefore, despite the greater costs, we believe that a custom-made prostheses should be the first choice, especially in young patients with large cranial defects due to results of decompressive craniotomy; in selected cases, however, precurved meshes may be recommended for small defects in particular, and for single- stage resection reconstruction procedure. In these situations, precurved mesh could be used with good results and with a simultaneous reduction in health-care costs.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Annan M, De Toffol B, Hommet C, Mondon K. Sinking skin flap syndrome (or syndrome of the trephined): A review. Br J Neurosurg. 2015. 29: 314-8
2. Baumeister S, Peek A, Friedman A, Levin LS, Marcus JR. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008. 122: 195e-208e
3. Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009. 26: E10-
4. Corliss B, Gooldy T, Vaziri S, Kubilis P, Murad G, Fargen K. Complications after in vivo and ex vivo autologous bone flap storage for cranioplasty: A comparative analysis of the literature. World Neurosurg. 2016. 96: 510-5
5. De Bonis P, Frassanito P, Mangiola A, Nucci CG, Anile C, Pompucci A. Cranial repair: How complicated is filling a hole?. J Neurotrauma. 2012. 29: 1071-6
6. De Bonis P, Pompucci A, Mangiola A, D’Alessandris QG, Rigante L, Anile C. Decompressive craniectomy for the treatment of traumatic brain injury: Does an age limit exist?. J Neurosurg. 2010. 112: 1150-3
7. Ehrlich G, Kindling S, Wenz H, Hänggi D, Schulte DM, Schmiedek P. Immediate titanium mesh implantation for patients with postcraniotomy neurosurgical site infections: Safe and aesthetic alternative procedure?. World Neurosurg. 2017. 99: 491-9
8. Fiaschi P, Pavanello M, Imperato A, Dallolio V, Accogli A, Capra V. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. J Neurosurg Pediatr. 2016. 17: 705-10
9. Fricia M, Nicolosi F, Ganau M, Cebula H, Todeschi J, Santin MD. Cranioplasty with porous hydroxyapatite custom-made bone flap: Results from a multicenter study enrolling 149 patients over 15 years. World Neurosurg. 2019. 121: 160-5
10. Gilardino MS, Karunanayake M, Al-Humsi T, Izadpanah A, Al-Ajmi H, Marcoux J. A comparison and cost analysis of cranioplasty techniques: Autologous bone versus custom computer-generated implants. J Craniofac Surg. 2015. 26: 113-7
11. Honeybul S, Morrison DA, Ho KM, Lind CR, Geelhoed E. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J Neurosurg. 2017. 126: 81-90
12. Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg. 2013. 71: e81-8
13. Joffe J, Harris M, Kahugu F, Nicoll S, Linney A, Richards R. A prospective study of computer-aided design and manufacture of titanium plate for cranioplasty and its clinical outcome. Br J Neurosurg. 1999. 13: 576-80
14. Jonkergouw J, van de Vijfeijken SE, Nout E, Theys T, Van de Casteele E, Folkersma H. Outcome in patient-specific PEEK cranioplasty: A two-center cohort study of 40 implants. J Craniomaxillofac Surg. 2016. 44: 1266-72
15. Kasprzak P, Tomaszewski G, Kotwica Z, Kwinta B, Zwoliński J. Reconstruction of cranial defects with individually formed cranial prostheses made of polypropylene polyester knitwear: An analysis of 48 consecutive patients. J Neurotrauma. 2012. 29: 1084-9
16. Kim BJ, Hong KS, Park KJ, Park DH, Chung YG, Kang SH. Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J Korean Neurosurg Soc. 2012. 52: 541-6
17. Kim JK, Lee SB, Yang SY. Cranioplasty using autologous bone versus porous polyethylene versus custom-made titanium mesh: A retrospective review of 108 patients. J Korean Neurosurg Soc. 2018. 61: 737-46
18. Kshettry VR, Hardy S, Weil RJ, Angelov L, Barnett GH. Immediate titanium cranioplasty after debridement and craniectomy for postcraniotomy surgical site infection. Neurosurgery. 2012. 70: 8-14
19. Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty-analysis by transcranial doppler ultrasonography. J Clin Neurosci. 2004. 11: 486-9
20. Lindner D, Schlothofer-Schumann K, Kern BC, Marx O, Müns A, Meixensberger J. Cranioplasty using custom-made hydroxyapatite versus titanium: A randomized clinical trial. J Neurosurg. 2017. 126: 175-83
21. Luo J, Liu B, Xie Z, Ding S, Zhuang Z, Lin L. Comparison of manually shaped and computer-shaped titanium mesh for repairing large frontotemporoparietal skull defects after traumatic brain injury. Neurosurg Focus. 2012. 33: E13-
22. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006. 148: 535-40
23. Policicchio D, Doda A, Sgaramella E, Ticca S, Santonio FV, Boccaletti R. Ultrasound-guided brain surgery: Echographic visibility of different pathologies and surgical applications in neurosurgical routine. Acta Neurochir (Wien). 2018. 160: 1175-85
24. Sakamoto S, Eguchi K, Kiura Y, Arita K, Kurisu K. CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg. 2006. 108: 583-5
25. Stefini R, Esposito G, Zanotti B, Iaccarino C, Fontanella MM, Servadei F. Use of custom made porous hydroxyapatite implants for cranioplasty: Postoperative analysis of complications in 1549 patients. Surg Neurol Int. 2013. 4: 12-
26. Van de Vijfeijken SE, Münker TJ, Spijker R, Karssemakers LH, Vandertop WP, Becking AG. Autologous bone is inferior to alloplastic cranioplasties: Safety of autograft and allograft materials for cranioplasties, a systematic review. World Neurosurg. 2018. 117: 443-52.e8
27. Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: Early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg. 2015. 44: 599-608
28. Wind JJ, Ohaegbulam C, Iwamoto FM, Black PM, Park JK. Immediate titanium mesh cranioplasty for treatment of postcraniotomy infections. World Neurosurg. 2013. 79: 207.e11-3