- Department of Otolaryngology, Royal Pearl Hospital, Tiruchirapally, Tamil Nadu, India
- Department of Neurosurgical Oncology, BIDMC Cancer Center, Boston, Massachusetts, USA
- Department of Neurological Surgery, COMS, Nepal
Correspondence Address:
Trichy N. Janakiram
Department of Neurological Surgery, COMS, Nepal
DOI:10.4103/sni.sni_295_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Trichy N. Janakiram, Shilpee B. Sharma, Ekkehard Kasper, Onkar Deshmukh, Iype Cherian. Comprehensive preoperative staging system for endoscopic single and multicorridor approaches to juvenile nasal angiofibromas. 26-Apr-2017;8:55
How to cite this URL: Trichy N. Janakiram, Shilpee B. Sharma, Ekkehard Kasper, Onkar Deshmukh, Iype Cherian. Comprehensive preoperative staging system for endoscopic single and multicorridor approaches to juvenile nasal angiofibromas. 26-Apr-2017;8:55. Available from: http://surgicalneurologyint.com/surgicalint-articles/comprehensive-preoperative-staging-system-for-endoscopic-single-and-multicorridor-approaches-to-juvenile-nasal-angiofibromas/
Abstract
Background:Juvenile nasal angiofibromas (JNA) is a benign lesion with high vascularity and propensity of bone erosion leading to skull base invasion and intracranial extension. It is known to involve multiple compartments, which are often surgically difficult to access. With evolution in surgical expertise and technical innovations, endoscopic and endoscopic-assisted management has become the preferred choice of surgical management. Over the last four decades, various staging systems have been proposed, which are largely based on the extent of nasal angiofibroma. However, no clear guidelines exist for the stage-appropriate surgical management. In this study, we aim to formulate a novel staging system based on the analysis of high quality preoperative imaging and propose detailed surgical guidelines related to disease stages as observed in 242 primary cases of JNA.
Methods:A retrospective analysis of the case records of 242 primary JNA cases was performed at our center. Patients were staged according to various existing staging systems as well as our own new staging system, and outcome variables were compared with respect to intraoperative blood loss, multiple staged operations, and tumor recurrences. Operative records were studied and precise endoscopic surgical guidelines were formulated for each stage.
Results:Comparing the intraoperative blood loss seen in stages of various classifications, it was found that intraoperative blood loss correlated best and statistically significantly with stages in the newly proposed Janakiram staging system when compared to the existing staging systems. Staged operations were performed in a total of 7/242 patients, and there was a significant association between the requirement of a staged operation and tumor extent (Fischer's exact test, P
Conclusion:This new Janakiram staging system is based on preoperative imaging data from one of the largest JNA case series reported thus far. Respective guidelines reliably stratify patients into treatment groups with definite surgical approaches and predicts outcome. Improved surgical approaches in the modern endoscopic era have redefined JNA management with improved outcome. This study shows the importance of precise presurgical imaging and the choice of the most suitable surgical approach in reducing morbidity and mortality in JNA surgery.
Keywords: Endoscopic excision, JNA, staging system
INTRODUCTION
Juvenile nasal angiofibromas (JNA) is a benign neoplastic lesion encountered in the anterior skull base, and is characterized by significant vascularity and a propensity of bone erosion with intracranial extension.[
Optimal imaging is an indispensable tool in the assessment and is the key element in planning surgery for JNA. Adequately classifying the stage and precise extent of such a distinct pathology is essential, and will determine the most suitable operative approach as well as the extent of resection that can be achieved. This in turn has profound implications on the prognosis.[
Any staging system must continuously be updated to reflect changes in our knowledge of tumor characteristics or our understanding of recurrence patterns and how these features relate to available treatment options.[
In the present study, we analyze preoperative records of 242 consecutive JNA patients treated at our center over a period of 9 years. Based on our experience, we propose and evaluate a new classification system that is derived from preoperative imaging data. Each stage in this classification system assigns patients to a specific treatment group with a defined surgical approach appropriate to address specific issues encountered in the respective anatomical compartment. Preoperative computed tomography (CT) data was analyzed in detail to especially understand the degree of tumor invasion into the skull base and beyond. With this classification-based surgical approach, we explore the suitability of exclusive single transnasal corridor surgery as well as endoscopic multicorridor approaches for these tumors. Based on our experience, we demonstrate that, with endoscopic multicorridor approaches, we can stretch the limits of endoscopic JNA resection to a new level. Only a small number of tumors that are very extensive will remain beyond management via such endoscopic-controlled multicorridor approaches, and should, in our opinion, be resected by endoscopic-assisted open approaches.
Furthermore, in this study, new insights were gained regarding the possible site of JNA origin and the common pathways of tumor spread, which can be inferred from observations made during surgery.
We discuss the implications of this assessment for the overall management strategies of JNA, and it will be shown that this classification clearly correlates to the prognosis of the patient.
MATERIALS AND METHODS
The entire medical and radiographic records of patients who underwent endoscopic resection of JNA at our center between January 2005 and January 2014 were retrospectively analyzed.
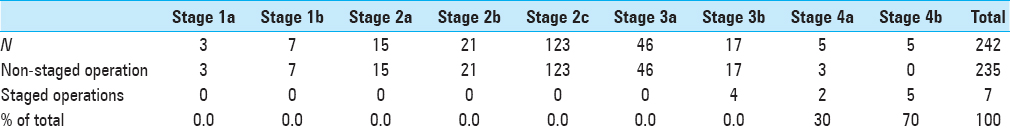
Two hundred and forty-two primary cases of JNA were identified for inclusion in this cohort analysis; all the included patients were young males between 10 and 25 years of age. Cases were categorized and assigned to groups according to the proposed Janakiram staging system (JSS). The JSS takes into account the sites of skull base involvement and the pattern of JNA extension based on preoperative imaging (see below). The most suitable approach and operative technique is then chosen according to this assigned stage.
Imaging
Preoperative evaluation included contrast-enhanced CT scan (0.625 mm) of the skull base and paranasal sinuses obtained in three-dimensional projections in both bone and soft tissue windowing. In advanced tumor cases with orbital, parapharyngeal, or intracranial extension, additional magnetic resonance (MR) imaging with contrast was obtained.
Patients with lesions in close proximity to or with involvement of the internal carotid artery (ICA) underwent additional conventional transfemoral angiography for identification of the tumor supplying vessels. Cases that involved the ICA to a significant degree underwent balloon occlusion testing to assess cross circulation. Preoperative embolization was not deemed necessary and hence not performed in any of our cases. Intraoperative navigation was used for all cases infiltrating the greater wing of sphenoid, tumors encasing the ICA, infiltrating the cavernous sinus, or extending intracranially. To avoid ICA injuries, microvascular Doppler sonography was used to localize the vessel intraoperatively.
Postoperative evaluation included a contrast-enhanced CT within 36 hours of surgery to identify any residual tumor burden. In cases in which any significant residual tumor mass was displayed, patients were taken back to the operation room electively for a second surgery session. In all cases of ICA transposition, a second conventional angiography was performed postoperatively.
Postoperative follow-up data included CT scanning immediately after resection, as described above, and at 6 months postoperatively, along with nasal endoscopic examinations every 3 months for the first year after surgery and every 6 months for the following 3 years as well as a comprehensive clinical exam at each visit. Additional scan was obtained if the endoscopy revealed any suspision for recurrence. Recurrent disease was defined as new tumor seen on subsequent imaging studies during the follow-up observation period after an initially negative postoperative scan.
Staging system
The JSS is based on tumor extent as seen on preoperative contrast-enhanced CT. It consists of 5 stages and substages as they relate to endoscopic surgery, as well as one separate advanced stage for rare JNA extensions, which are beyond the access of endoscopic approaches. Our staging system advocates a tailored surgical strategy for each anatomical tumor configuration depending upon the various compartments involved. The staging system employed at our center for JNA is presented in
Figure 1
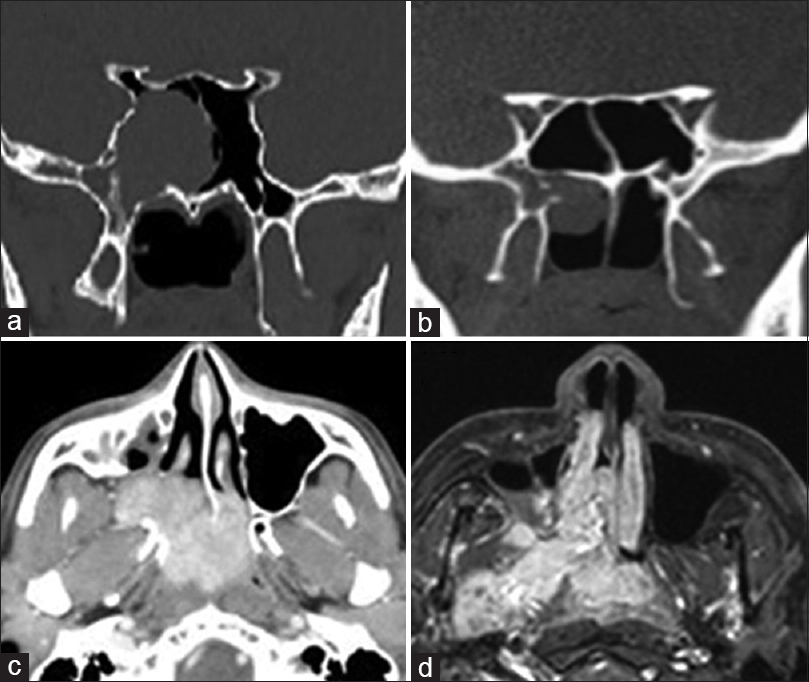
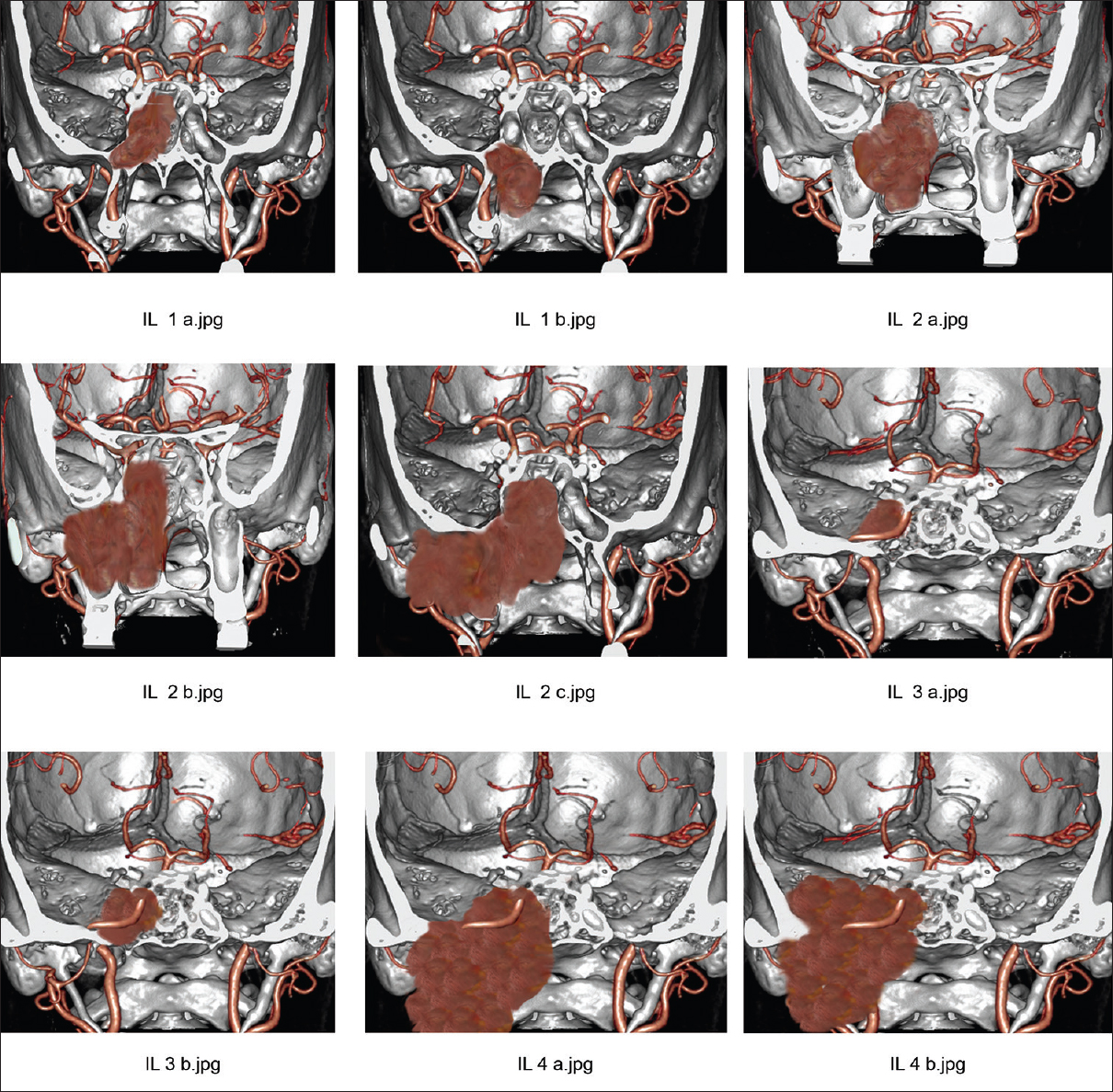
Preoperative imaging in Janakiram staging system. (a) Tumor seen involving pterygoid wedge and paranasal sinus on a coronal section CT scan (Stage 1a). (b) On a coronal section CT scan, tumor seen extending to the nasopharynx (Stage 1b). (c) Axial section CT scan showing tumor extension to the nasal cavity and pterygopalatine fossa (Stage 2a). (d) Tumor seen extending to the infratemporal fossa in an axial section (Stage 2b)
Figure 3
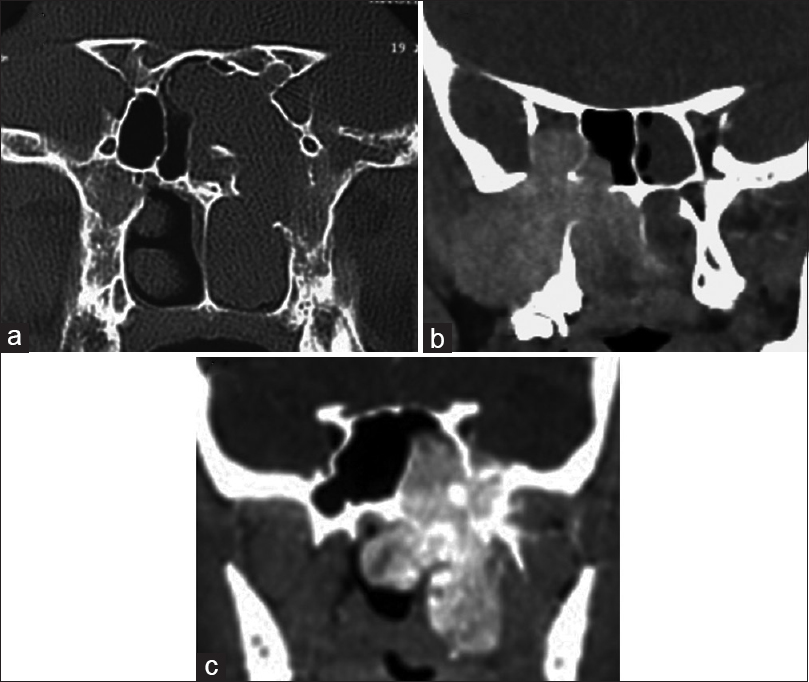
Preoperative imaging in Janakiram staging system. (a, b) Axial and coronal views of tumor in the Meckel's cave (Stage 3a). (c) Axial cut showing tumor engulfing the internal carotid artery (Stage 3b). (d) Tumor seen extending in to the parapharyngeal space in a coronal section (Stage 4a). (e, f) Coronal and axial sections showing intracranial tumor extension in to the middle cranial fossa (Stage 4b)
Figure 4
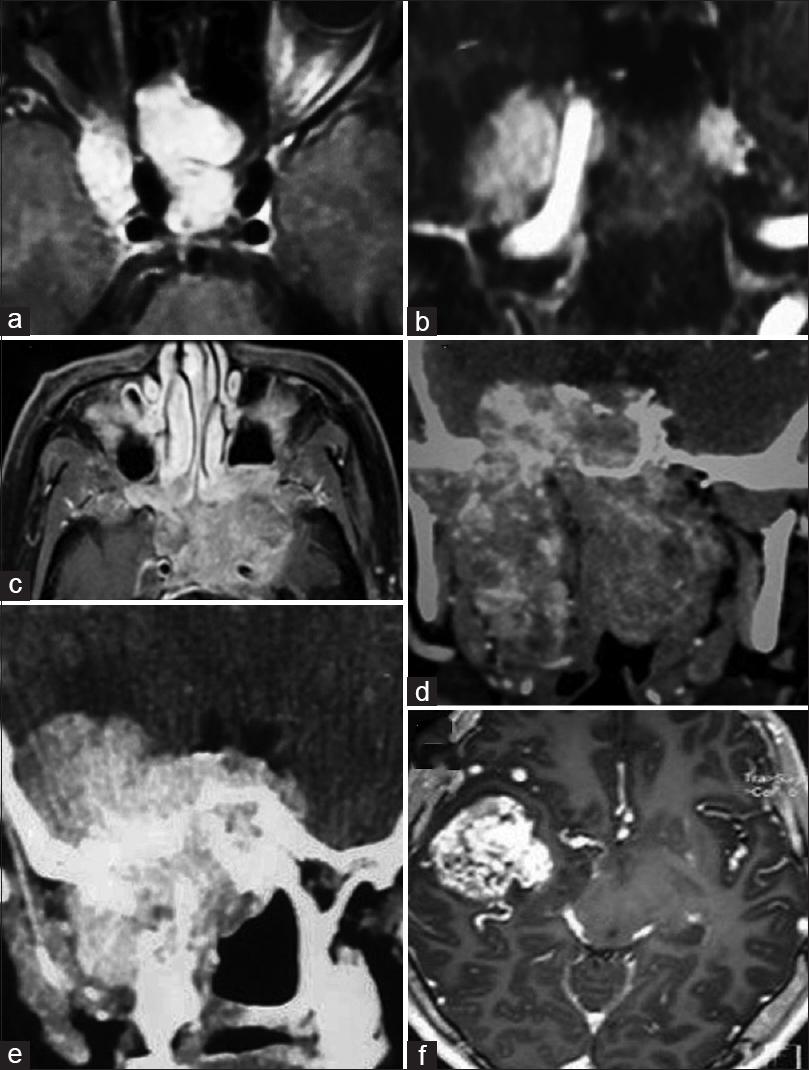
Postoperative CT scans. (a) postoperative CT scan of a Stage 1a. (b) similar postoperative scans of stage 2b. (c) Stage 2c with extension in the IOF. (d) Postoperative CT scan stage 2c with extension to the pterygoid fossa. (e) Postoperative CT scan of stage 2c with extension to cheek. (f) Postoperative CT scan of a stage 3b JNA. (g) Postoperative CT scan of a stage 4a JNA
Figure 5
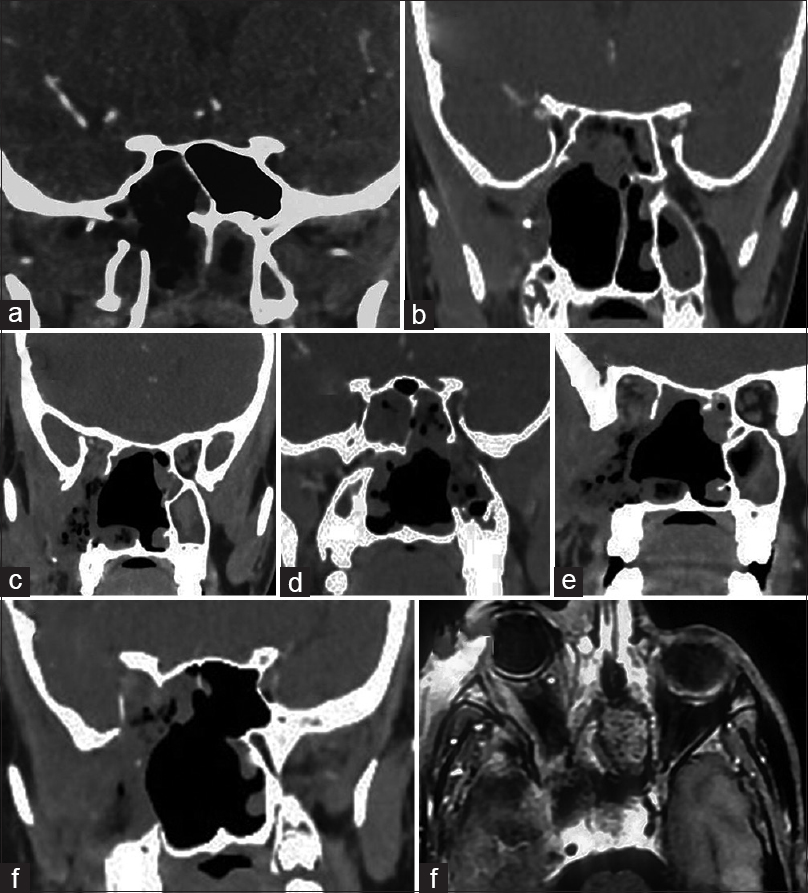
Stage 1a – Coronal cut section showing JNA in the pterygoid wedge area and occupying the sphenoid sinus. Stage 1b – JNA in the pterygoid wedge area and extending to the nasopharynx. Stage 2a – JNA extending in the nasal cavity and minimal involvement of pterygopaltine fossa. Stage 2b – JNA extending to the pterygopalatine fossa. Stage 2c – JNA involving the infratemporal fossa. Stage 3a – JNA extending to the quadrangular space. Stage 3b – JNA engulfing the internal carotid artery. Stage 4a – JNA extending to the Prestyloid parapharyngeal space. Stage 4b – JNA extending intracranially
Surgical planning
General transoral endotracheal anesthesia was administered. Hypotensive anesthesia (htA) was maintained using a nitroglycerine infusion. Goal in hypotensive anesthesia is to achieve mean arterial pressures of 60–70 mmHg and systolic blood pressure values at about 80–90 mm Hg. Hemodynamics were closely monitored with invasive access throughout the case. The four-handed binostril technique was used in all cases except in stage 1 cases.
In the following paragraph [
Statistical analyses
A number of variables (tumor extension based on presurgical imaging, surgical approaches chosen, direct vascular control by ligation of the feeder vessels, intraoperative blood loss, occurrence of residual or recurrent disease, and need for staged surgery) was analyzed for tumor staging and subsequently correlated to clinical outcome.
The database of our cohort was also used to classify our patient series according to other already established staging systems.[
RESULTS
Demographics
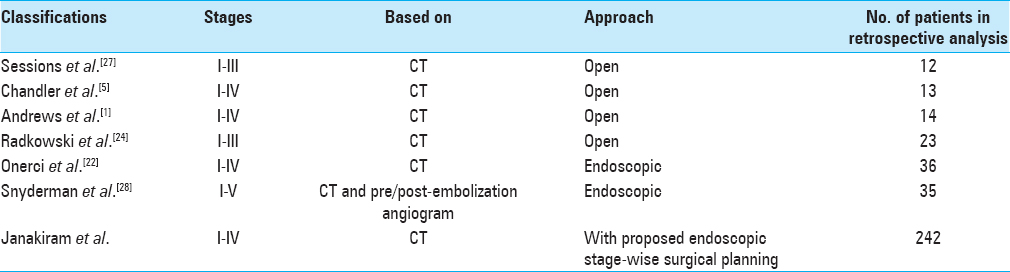
Between January 2005 and January 2014, a total of 242 JNA patients were identified as requiring surgery and were subsequently operated in our department. All patients were males. The mean age at surgery was 17.3 years (range = 10–25 years). All patients were fully worked up and their tumor was categorized according to the JSS. A comparison of the JSS to other existing staging systems is presented in
Imaging
On analysis of preoperative CT imaging studies of the entire cohort of 242 native JNA cases, the pterygoid wedge and floor of sphenoid sinus were the most commonly involved skull base structures. On further detailed analysis of the imaging studies, it was noted that the epicenter of the tumor could be localized to the pterygoid wedge in all our cases. The vidian canal was identifiable as a separate structure in 19% of our JNA cases.
The nasopharynx and the pterygopalatine fossa were involved in 98.4% and 95.8% of the cases, respectively. The tumor was observed to frequently extend via an anterolateral pathway reaching into the infratemporal fossa in 89.66% of the cases. Once tumor was present in the infratemporal fossa, it showed multidirectional spread along pathways of low resistance.
Orbital extension through the inferior orbital fissure was seen in 25.6% of the cases. This pathway was typically also associated with involvement of the infratemporal fossa. The pterygoid fossa was involved in 21.9% of the cases in our patient cohort. The greater wing of sphenoid was involved in 45.8% of the patients. Finally, we observed that the tumor extended from the pterygoid wedge along the greater wing of sphenoid into the middle cranial fossa in 2.1% of the patients.
A second typical pattern observed was that of the tumor extending posteriorly towards the quadrangular space with erosion of the base of pterygoid and the greater wing of the sphenoid. The quadrangular space was found to be involved in 30.1% of the cases. Tumors extending into the quadrangular space were located anterior to the anterolateral wall of cavernous sinus. Involvement of the cavernous sinus itself was quite rare and seen in only 6 cases. The most common segment of the ICA, which showed some tumor involvement was the paraclival ICA. Please refer to
Residual and recurrent tumor
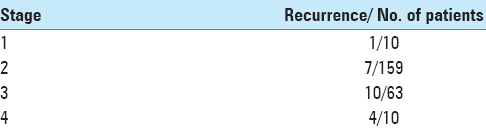
Out of the entire cohort of 242 cases, we observed 19 cases with residual disease and 22 recurrent cases
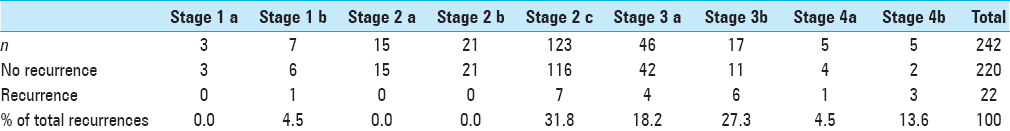
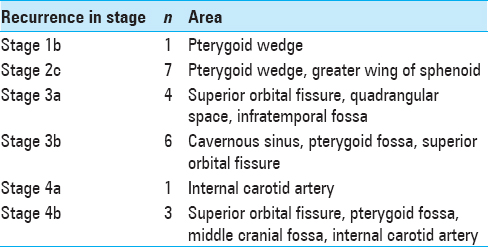
The various sites of observed tumor recurrence are listed in
Residual tumor was found in only 19/242 cases on the initial (36 hour) postoperative scan, and patients who showed residual tumor burden, underwent a second surgery for excision. The group with residual disease constituted 7.8% of the JNA cases of this series operated at our center.
Staged operation
Seven patients underwent surgery that was planned as a two-step operation to begin with owing to the anticipated profound intraoperative blood loss during the primary surgery. There was a statistically significant correlation between the extent (stage) of the tumor on presurgical imaging and the need for staging the procedure into multiple operations. (fischer exact test P < 0.001) Please see
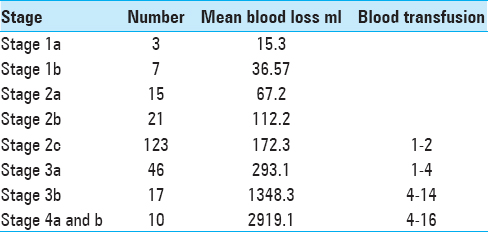
Intraoperative blood loss
Intraoperative blood loss is an important outcome of surgery and helps to evaluate efficacy of a surgical approach.[
A clear progressive increase in intraoperative blood loss was observed with higher stages of disease/more advanced disease. The correlation of blood loss with extent of disease (stage) was studied for the newly proposed JSS as well as for other already existing staging systems; it became evident that blood loss correlated best with the stages in the JSS staging system when compared to others (r = 0.92 by Spearman rank correlation;
DISCUSSION
Owing to its dense vascularity and rather aggressive growth, JNA is a very challenging tumor – even for experienced surgeons. However, over the last years, a striking paradigm shift in surgical management of JNA has occurred with new endoscopic and endoscopic-assisted approaches performed by expert hands. With superior surgical instrumentation available, better endoscopic illumination techniques and substantially better direct vascular control methods, complete tumor removal can now be achieved for even very advanced cases of JNA, as seen in our stages 3a and above.
The primary objective of a clinically meaningful tumor staging system is to aid in determining the best management protocol as well as to prognosticate treatment outcome. After the introduction of the first classification system by Sessions et al. in 1981, a number of modifications were proposed.[
Our staging system is predominantly pathoanatomical and classifies tumors based on their topographic extension, as seen on high-resolution preoperative imaging. It divides JNA into distinct subgroups that represent progressively more extensive tumor stages, and pairs each with a most suitable surgical approach for definite care. This system is based on the surgical principle of to distinct subgroups that represent progressively more extensive tumor stages, and pairs each with a most suitable surgical approach for definite care. This system is based on the surgical principle of “centripetal dissection” and sequential segmental resection.
The tailored approach evolved in our institution over time and its applicability can be seen in the analysis of this extensive cohort of 242 JNA patients who were operated strictly endoscopically or with endoscopically-assisted techniques at our center over the past 9 years.
A detailed preoperative CT scan analysis revealed the pterygoid wedge to be the most common site of residual tumor during the early phase of our surgical series. This observation was made on follow-up CT, and it was focally addressed endoscopically during the next cases as the surgical technique evolved and as we became more radical in our resection strategy. This knowledge was also applied to a small set of patients undergoing repeat surgeries. As we reinspected the pterygoid area, additional tumor could be identified at the pterygoid wedge, and more extensive “in depth” drilling was performed for all these cases. This led to no residual/recurrent tumor in this area on subsequent CT imaging. In three cases, we observed that JNA was limited to the pterygoid wedge and sphenoid on high-resolution CT without any nasopharyngeal soft tissue involvement at all on endoscopy. This observation was confirmed repeatedly in our growing series and with longer follow-up intervals, thereby revealing that the pterygoid wedge functions as the most likely site of origin of the tumor.
It is our impression that tumor growth occurs along certain tissue planes, which are those of least resistance. Another mode of spread appears to be along the vascular channels in the nose and skull base. The pathway of progressive growth and extension of tumor mass with bone destruction in JNA is more a “pushing” pattern of growth rather than one of malignant infiltration and this explains that the tumor – when spreading intracranially – respects the barrier formed by the dura.[
The tumor extension seen preoperatively on CT scan here was correlated to what we saw endoscopically, and both modes of observation appeared congruent in their findings.
Therefore, it was concluded that a designated preoperative CT protocol of high quality and thin cut technique for both bone and soft tissue windowing can help the surgeon to develop a clear strategy of how to approach this unique tumor in a stepwise manner. Once the tumor pattern and extent can be fully appreciated, the surgical team merely has to follow the proposed technical steps and principles.
This image-based staging system emphasizes the necessity of an accurate preoperative assessment of the lesion to truly understand the extent of tumor infiltration. It also allows to pay tribute to extensions into difficult to reach anatomical areas, which then establishes the need to approach such extensive tumors via multiple surgical corridors or the rare need for a planned staged resection.
Blood loss is one of the most important outcome variables in JNA resections and a parameter which depends not only on surgical skill but largely on surgical strategies and stepwise surgical progress made intraoperatively.[
We found a consistent and positive correlation between the observed tumor stage and the amount of blood loss encountered across each of the stages of our staging system. However, this finding is not in agreement with the observation by Snyderman et al. who found no consistent correlation between some staging systems and the respective blood loss.[
It is generally accepted that the pterygoid wedge represents a watershed zone for blood supply of JNA feeder vessels. This observation is reflected in our strategy of a segmental resection, which follows the principle that tumor feeding vessels anterior to the pterygoid wedge can be well controlled in a single step by direct visualization and ligation. That is also the reason that we did not pursue embolization preoperatively. This strategy will help avoid unwanted neurological sequelae that may be caused by embolization. The tumor posterior can be further resected in a staged approach depending on the blood loss encountered. We observed that, here again, blood loss can be managed well by direct surgical ligation without increasing the procedural morbidity and without the need for embolization.
Preoperative embolization has shown a reduction in intraoperative blood loss and a decrease in operative time in some studies, which will both facilitate complete excision.[
Another aspect that should be taken into account is that preoperative embolization is not without complications. Some major complications may occur such as common iliac artery thrombosis, acute pulmonary edema, and cranial nerve deficits. Other minor complications such as sore throat, hemifacial pain, temporomandibular joint sourness, nasal alar necrosis, fever, and periorbital pain have also been reported.[
In a noteworthy study by Moulin et al., no significant difference was noted with respect to intraoperative bleeding in JNA cases when embolized and nonembolized groups were compared.[
At our center, JNA cases were surgically treated without embolization, and intraoperative bleeding was addressed and managed by a multipronged strategy. The administration of hypotensive anesthesia was found to help in decreasing intraoperative blood loss. External carotid artery ligation can be performed in high-staged tumors. Intraoperatively, control over tumor feeding vessels was achieved by diligent visualization with direct identification and ligation, and those in close proximity of the ICA were cauterized using Kassam Bipolar forceps. Our surgical technique follows the principle of a “centripetal dissection,” which enables the surgeon to clearly identify the feeder vessels. Other maneuvers such as optimal positioning of the patient, intraoperative warm saline irrigation, and the application of hemostatic agents also contributed to the control of blood loss.
The assignment to a high stage in our system correlated well with the need of multiple-staged operations and was found to be another robust variable. This aided significantly in surgical planning and was also a relevant aspect of presurgical patient counseling. Operations for extensive and high-staged tumors were then broken down into multiple procedures in view of anticipated excessive intraoperative blood loss in the primary surgery, and a second surgery was planned to achieve complete tumor excision. There was a statistically significant correlation between the stage of the tumor and the need to stage the operation as per the JSS (P < 0.001).
Surgical strategy is of paramount importance in managing JNA and significantly affects the prognosis of this disease. Surgical confidence has increased with better anatomical orientation skills and with increased experience in endoscopic skull base tumor resections. Irrespective of a particular surgical strategy or trajectory, the goal of all surgical approaches is to achieve wide exposure and good visibility for adequate vascular control and minimize morbidity.[
The most important outcome, besides the patients’ clinical status in the postoperative period, is the frequency of observed tumor recurrences, which are often due to inaccessible locations or under-resected tumor margins due to high vascularity.[
JNA has a high propensity for recurrence with rates reported as high as 32%.[
Owing to this high recurrence rate seen in some JNA, a thorough and radical excision of the tumor with all its extension should be attempted. Boghani et al. stated in a comprehensive systematic review that, in purely endoscopic approaches, the recurrence rate ranges from only 0–23%. This number increases to 15–50% and 0–0% for endoscopic assisted and open approaches, respectively.[
As an endoscope provides a magnified and multiangled viewing opportunity, tumor extensions can be much better visualized than with a microscopic approach, and we think that this aspect is a key feature in explaining why the incidence of residual disease has been drastically reduced in well-done endoscopic cases.[
In this article we have made a sincere effort to propose a clinically meaningful JNA staging system, which correlates to clear operative guidelines that show that most JNA can be managed endoscopically. Using these approaches and the currently available endoscopic technology, we have been able to reach as far as the middle cranial fossa dura, parapharyngeal space, cheek, and temporal fossa. We believe that we have stretched the limits of minimal invasive resectability with such single or multicorridor endoscopic approaches. However, even in the most experienced hands, a few highly extensive tumors cannot be accessed endoscopically alone owing to their location and topography. For such tumors, we suggest to continue to employ external approaches, as recommended in the past.[
CONCLUSION
Surgical approaches in the endoscopic era have revolutionized the management of JNA and have improved surgical outcomes for extensive lesions. This study shows the importance of imaging in selecting the most suitable surgical approach with results in reducing morbidity and mortality in JNA surgery.
To this end we propose in this paper the JSS that is based on preoperative imaging characteristics obtained from data of one of the largest JNA case series to date. It reliably stratifies patients into treatment groups with definite surgical approaches, which redefine the achievable boundaries of endoscopic resection of JNA in complex areas such as Meckel's cave, cavernous sinus, and ICA. It also aids in predicting outcomes. It is of further value as it reports unique observations regarding presumed tumor origin and how this lesion spreads along preferred pathways.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrews JC, Fisch U, Valavanis A, Aeppli U, Makek MS. The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope. 1989. 99: 429-37
2. Boghani Z, Husain Q, Kanumuri VV, Khan M N, Sangvhi S, Liu JK. Juvenile nasopharyngeal angiofibroma: A systematic review and comparison of endoscopic, endoscopic- assisted, and open resection in 1047 cases. Laryngoscope. 2013. 123: 859-69
3. Carrillo JF, Maldonado F, Albores O, Ramírez-Ortega MC, Oñate-Ocaña LF. Juvenile nasopharyngeal angiofibroma: Clinical factors associated with recurrence, and proposal of a staging system. J Surg Oncol. 2008. 98: 75-80
4. Chandler JR, Goulding R, Moskowitz L, Quencer RM. Nasopharyngeal angiofibromas: Staging and management. Ann Otol Rhinol Laryngol. 1984. 93: 322-9
5. Dale BJ, Jakob SL. Temporal approach for resection of Juvenile Nasal angiofibroma. Laryngoscope. 2000. 110: 1287-92
6. Douglas R, Wormald PJ. Endoscopic surgery for juvenile nasopharyngeal angiofibroma: Where are the limits?. Curr Opin Otolaryngol Head Neck Surg. 2006. 14: 1-5
7. Enepekide DJ. Recent advances in the treatment of juvenile angiofibroma. Curr Opin Otolaryngol Head Neck Surg. 2004. 12: 495-9
8. Hofmann T, Bernal-Sprekelsen M, Koele W, Reittner P, Klein E, Stammberger H. Endoscopic resection of juvenile angiofibroma—Long term results. Rhinology. 2005. 43: 282-9
9. Kasemsiri P, Prevedello DM, Ditzel L, Otto BA, Old M, Teknos T. Surgical Treatment of Nasopharyngeal Malignancies: Role of Endoscopic Endonasal Approaches. J Nasopharyngeal Carcinoma. 2014. 1: e14-
10. Khoueir N, Nicolas N, Rohayem Z, Haddad A, Abou Hamad W. Exclusive endoscopic resection of juvenile nasopharyngeal angiofibroma: A systematic review of the literature. Otolaryngol Head Neck Surg. 2014. 150: 350-8
11. Li JR, Qian J, Shan XZ, Wang L. Evaluation of the effectiveness of preoperative embolization in surgery for nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol. 1998. 255: 430-2
12. Lloyd G, Howard D, Phelps P, Cheesman A. Juvenile angiofibroma: The lessons of 20 years of modern imaging. J Laryngol Otol. 1999. 113: 127-34
13. McCombe A, Lund VJ, Howard DJ. Recurrence in juvenile angiofibroma. Rhinology. 1990. 28: 97-102
14. Midilli R, Karci B, Akyildiz S. Juvenile nasopharyngeal angiofibroma: Analysis of 42 cases and important aspects of endoscopic approach. Int J Pediatr Otorhinolaryngol. 2009. 73: 401-8
15. Moulin G, Chagnaud C, Gras R, Gueguen E, Dessi P, Gaubert JY. Juvenile nasopharyngeal angiofibroma: Comparison of blood loss during removal in embolized group versus nonembolized group. Cardiovasc Intervent Radiol. 1995. 18: 158-61
16. Nicolai P, Berlucchi M, Tomenzoli D, Capiello J, Trimarchi M, Maroldi R. Endoscopic surgery of Juvenile angiofibroma: When and how. Laryngoscope. 2003. 113: 775-82
17. Nicolai P, Villaret AB, Farina D, Nadeau S, Yakirevitch A, Berlucchi M. Endoscopic surgery for juvenile angiofibroma: A critical review of indications after 46 cases. Am J Rhinol Allergy. 2010. 24: e67-e72
18. Nicolai P, Schreiber A, Bolzoni Villaret A. Juvenile nasopharyngeal angiofibroma: Evolution of management. Int J Pediatrics 2012. 2012. p.
19. Ogawa AI, Fornazieri MA, da Silva LV, Pinna FR, Voegels RL, Sennes LU. Juvenile angiofibroma: Major and minor complications of preoperative embolization. Rhinology. 2012. 50: 199-202
20. Onerci M, Oğretmenoğlu O, Yücel T. Juvenile nasopharyngeal angiofibroma: A revised staging system. Rhinology. 2006. 44: 39-45
21. Onerci M, Gumus K, Cil B, Eldem B. A rare complication of embolization in juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol. 2005. 69: 423-8
22. Onerci TM, Yucel OT, Ogretmenoglu O. Endoscopic surgery in the treatment of juvenile nasopharyngeal angiofibroma. Int J Pediatr Otolaryngol. 2003. 67: 1219-25
23. Pryor SG, Moore EJ, Kasperbauer JL. Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma. Laryngoscope. 2005. 43: 282-9
24. Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT. Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg. 1996. 122: 122-9
25. Ramezani A, Haghighatkhah H, Moghadasi H, Taheri MS, Parsafar H. A case of central retinal artery occlusion following embolization procedure for juvenile nasopharyngeal angiofibroma. Indian J Ophthalmol. 2010. 58: 419-21
26. Renkonen S, Hagström J, Vuola J, Niemelä M, Porras M, Kivivuori SM. The changing surgical management of juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol. 2011. 268: 599-607
27. Roger G, Tran Ba Huy P, Froelich P, Van Den Abbeele T, Klossek JM, Serrano E. Exclusively endoscopic removal of juvenile nasopharyngeal angiofibroma: Trends and limits. Arch Otolaryngol Head Neck Surg. 2002. 128: 928-35
28. Sessions RB, Bryan RN, Naclerio RM, Alford BR. Radiographic staging of juvenile angiofibroma. Head Neck Surg. 1981. 3: 279-83
29. Snyderman CH, Pant H, Carrau RL, Gardner P. A new endoscopic staging system for angiofibromas. Arch Otolaryngol Head Neck Surg. 2010. 136: 588-94
30. Trivedi M, Desai RJ, Potdar NA, Shinde CA, Ukirde V, Bhuta M. Vision Loss due to Central Retinal Artery Occlusion Following Embolization in a Case of a Giant Juvenile Nasopharyngeal Angiofibroma. J Craniofac Surg. 2015. 26: e451-3