- San Juan Bautista School of Medicine, Caguas, Puerto Rico, USA
- Caribbean Neurological Center, Guaynabo, Puerto Rico, USA
Correspondence Address:
Sara Zarei
Caribbean Neurological Center, Guaynabo, Puerto Rico, USA
DOI:10.4103/sni.sni_224_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Sara Zarei, James Eggert, Laura Franqui-Dominguez, Yonatan Carl, Fernando Boria, Marina Stukova, Alessandro Avila, Cristina Rubi, Angel Chinea. Comprehensive review of neuromyelitis optica and clinical characteristics of neuromyelitis optica patients in Puerto Rico. 03-Dec-2018;9:242
How to cite this URL: Sara Zarei, James Eggert, Laura Franqui-Dominguez, Yonatan Carl, Fernando Boria, Marina Stukova, Alessandro Avila, Cristina Rubi, Angel Chinea. Comprehensive review of neuromyelitis optica and clinical characteristics of neuromyelitis optica patients in Puerto Rico. 03-Dec-2018;9:242. Available from: http://surgicalneurologyint.com/surgicalint-articles/9103/
Abstract
Neuromyelitis optica (NMO) is an immune-mediated inflammatory disorder of the central nervous system. It is characterized by concurrent inflammation and demyelination of the optic nerve (optic neuritis [ON]) and the spinal cord (myelitis). Multiple studies show variations in prevalence, clinical, and demographic features of NMO among different populations. In addition, ethnicity and race are known as important factors on disease phenotype and clinical outcomes. There are little data on information about NMO patients in underserved groups, including Puerto Rico (PR). In this research, we will provide a comprehensive overview of all aspects of NMO, including epidemiology, environmental risk factors, genetic factors, molecular mechanism, symptoms, comorbidities and clinical differentiation, diagnosis, treatment, its management, and prognosis. We will also evaluate the demographic features and clinical phenotype of NMO patients in PR. This will provide a better understanding of NMO and establish a basis of knowledge that can be used to improve care. Furthermore, this type of population-based study can distinguish the clinical features variation among NMO patients and will provide insight into the potential mechanisms that cause these variations.
Keywords: AQP4 antibodies, multiple sclerosis, myelitis, neuromyelitis optica, optic neuritis
INTRODUCTION
In 1894, Eugène Devic and his student Fernand Gault created the term neuromyelitis optica (NMO) in a published review of early cases of patients that presented with bilateral optic neuritis (ON) and myelitis accompanied by debilitating disability after series of attacks.[
In 2015, the International Panel for NMO Diagnosis proposed the unifying term of neuromyelitis optica spectrum disorders (NMOSD) for patients presenting selective demyelination of the spinal cord and the optic nerve. Specific criteria were established to facilitate earlier and more accurate diagnoses in AQP4 antibodies seropositive or seronegative patients presenting with ON, transverse myelitis, or area postrema clinical syndrome associated with a medullary MRI lesion.[
In the following sections, we will provide a comprehensive review of NMO that covers its epidemiology, environmental risk factors, genetic factors, molecular mechanism, symptoms, comorbidities and clinical differentiation, diagnosis, treatment, its management, and prognosis.
In addition, we also provide information specific to NMO patients in Puerto Rico (PR).
A recent study on Latin Americans with NMO (including patients from Caribbean islands) found the prevalence of NMO in this population ranged from 0.37/100,000 to 4.2/100,000 with African, Brazilian young women having the highest frequency.[
We will evaluate contributing factors and clinical phenotype of NMO patients in PR. This type of population-based study can help the treating physicians with understanding the prognosis of NMO among this specific population. Furthermore, it will enhance their knowledge on the variability of the disease that is based on environmental factors and ethnicity. We believe that this research can delineate the clinical features variation among NMO patients and will provide insight into the potential mechanisms behind these variations.
EPIDEMIOLOGY OF NEUROMYELITIS OPTICA
The evolution in the knowledge of NMO and the changes in the selection criteria have influenced the incidence and prevalence rates reported in many countries, with prevalence ranging from 0.05 to 4.4/100,000 worldwide.[
In some countries, the studies for diagnosing NMO are scarce and only an average age of onset for the disease is reported, such as in Algeria with a mean of 29.4 years old (range 16–44 years) or Iran with a mean of 36.6 years old (mode of 30).[
For many years, NMO has been associated with Indian, black, and Asian populations: the Japanese population, for example, has one of the highest documented prevalence of NMO in the world at 3.4/100,000.[
Studies in Latin America show patterns of higher relative frequency of NMO in relation with MS in populations with a higher presence of non-white descendants. In Buenos Aires, Argentina, the lowest relative frequency is reported at 2.1% in a population of high European descent. Paraguay, composed of 30% of mestizos population, reports a frequency of 8.7%. Caracas, Venezuela, reports the highest relative frequency of NMO at 11.8% with the highest non-white presence 79.15%.[
Female preponderance is reported in NMO patients, in both pediatrics and adults.[
ENVIRONMENTAL RISK FACTORS IN NEUROMYELITIS OPTICA
The association between environmental factors with the incidence of autoimmune diseases is well studied over many years.[
Gastrointestinal
Many studies look for the association between diet and gastrointestinal infections with prevalent diseases in the Asian population.[
Clostridium perfringens (C. perfringens) is also found to be one of the most active bacteria in NMO patients microbiota.[
Studies regarding the change in climate and seasonal viral infections in Japan showed an association with relapses in MS patients but not in NMO patients.[
Vitamin D
Though the importance of active vitamin D, or 1,25-dihydroxycholecalciferol, in immune system function is well known, it's role in the pathogenesis of autoimmune-mediated demyelinating neuropathies is only now just starting to be understood. Vitamin D acts to increase regulatory T cell (Treg) function via increased interleukin 10, as well as suppression of interferon Υ, interleukin 2, and, at least in-vitro, interleukin 4.[
Vitamin D synthesis is dependent on ultraviolet (UV) light and is needed for adequate immunologic responses.[
Additional studies show a correlation between both NMO and MS and vitamin D deficiency, though neither study found correlation between either prevalence nor degree of vitamin D deficiency and annualized relapse rates of NMO.[
Other investigators report independent roles for the amount of vitamin D in the body and history of sun exposure on demyelinating disorders and this is an area in need of further study.[
Smoking
One study reports that smoking is more frequent in MS than NMO for two case cohorts, Canadian (37.5% vs. 10.5%, P = 0.039) and Chinese (14.5% vs. 0%, P = 0.004).[
Other
Determinant and protective factors like breastfeeding and daycare exposure are also reported in different populations for NMO patients.[
GENETIC RISK FACTORS IN NEUROMYELITIS OPTICA
Genetic predisposition of NMO to different alleles of Class I and II human leukocyte antigen (HLA) has been studied since NMO was considered a variant of MS. Studies performed before 2001 on natives of Canada with demyelinated lesions in optic nerve and cervical spinal cord showed association of these lesions with HLA alleles DRB1 and DQB1.[
In France, no association is found between NMO patients and HLA-DR-DPB1*05:01; nevertheless, a different investigation found this allele in NMO patients of African-American and Latino population.[
Protective factors
In Asian populations, AQP4 Ig seropositive or seronegative HLA-DRB1*0901 is protective against NMO.[
Genes associated with AQP4
Studies in Chinese patients found the 3′ UTR region of AQP4 gene to be susceptible in several sites to SNP that may represent a risk for NMO.[
In AQP4-Ig-positive Japanese patients, the allele frequency of T rs2075575 is higher than in controls (50% vs. 25.7%; P = 0.0036) and represents higher risk for AQP4-Ig NMO.[
Other genes associated with neuromyelitis optica
Genes involved in regulation of the autoimmune system and regulation processes are found in NMO patients with lesions.[
MOLECULAR MECHANISM OF NEUROMYELITIS OPTICA
Antibody mediated damage
The major pathological mechanism of injury in seropositive NMO involves the autoantibodies aquaporin-4 (AQP4-IgG) binding to aquaporin-4 water channels in the astrocytes of brain, spinal cord, and optic nerve, followed by inflammation, disruption of blood–brain barrier, and complement-dependent cytotoxicity.[
Role of complement
The MAC-inhibitory protein, CD59 is responsible for protection from membrane attack complex (MAC) formation in AQP4-expressing tissues in the periphery of seropositive NMO patients.[
T-cell-mediated damage
T cells may also play an important role. T cells from mice lacking AQP4 channels recognize AQP4 epitopes, unlike the T cells of wild-type mice.[
Other mechanisms
Autoantibodies against myelin–oligodendrocyte glycoprotein, a membrane protein expressed on the oligodendrocyte cell surface and the outermost surface of myelin sheaths, are found in AQP4-IgG-seronegative patients who meet the diagnostic criteria for NMO.[
SYMPTOMS OF NEUROMYELITIS OPTICA
The international criteria for the diagnosis of NMOSD include core clinical characteristics related to the optic nerve, spinal cord, area postrema, brainstem, diencephalic, or cerebral presentations.[
CLINICAL DIFFERENTIATION AND COMORBIDITIES OF NEUROMYELITIS OPTICA
Neurological pathologies present from several etiologies and the clinician must be able to identify subtle differences between the presentations by taking a detailed medical history.[
The predominant symptom in NMOSD is ON. As NMO and NMOSD are recent classifications apart from MS, the presentations between NMOSD and MS are similar. ON as a visual defect other than cecocentral scotoma and severe vision loss in the chronic stage occurs more frequently in NMOSD than in MS, as do the brainstem symptoms such as hiccup and nausea, myelitis with neuropathic pain, complete paraplegia, and tonic spasms.[
Idiopathic ON can be considered if the clinical presentation of relapsing disease course, bilateral simultaneous nerve involvement, and poor visual outcomes of NMOSD are absent.[
NMOSD is commonly associated with autoimmune disorders.[
In the limited research into the concurrent cases of myasthenia gravis (MG) and NMOSD, AQP4-NMOSD is recognized to be associated with other autoimmune manifestations in 25–50% of cases, suggesting that patients with NMOSD and MG have a predisposition to autoimmune disorders.[
Neuro Behçet's disease is a subset of Behçet's disease involving the CNS and can resemble longitudinally extensive transverse myelitis (LETM) of NMOSD.[
Invasive etiologies may also be considered; NMOSD can be misdiagnosed for primary CNS lymphoma; diagnostic studies are important to differentiate the two. CNS lymphoma should be considered in patients with LETM if they continue to worsen despite treatment.[
Similarly, sarcoidosis is a systemic granulomatous disease that can also involve the spinal cord and optic nerve resembling phenotypes of NMOSD.[
Another process that can be considered in the differential is a spinal dural arteriovenous fistula. A spinal dural arteriovenous fistula is a vascular malformation in spinal cord that can present as subacute and progressive myelopathy; symptoms appear after exercise or prolonged rest.[
An additional cause of neurological disorders is infection. Syphilis is a sexually transmitted infection that can cause ON that can mimic NMOSD.[
Finally, Leber hereditary optic neuropathy is the most common hereditary ON affecting males in the second or third decade of life due to mutations in the mitochondria and can be considered in the differential.[
DIAGNOSIS OF NEUROMYELITIS OPTICA
With improved sensitivity and specificity of immunoassay techniques, as well as a deeper understanding of the pathogenesis of the disease, NMOSD diagnosis criteria have appreciably evolved over the past two decades.[
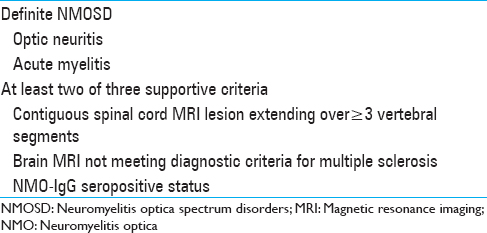
Table 1
Neuromyelitis Optica Spectrum Disorders Diagnostic Criteria, Wingerchuk et al.[
Table 2
Neuromyelitis Optica Spectrum Disorders Revised Diagnostic Criteria, Wingerchuk et al.[
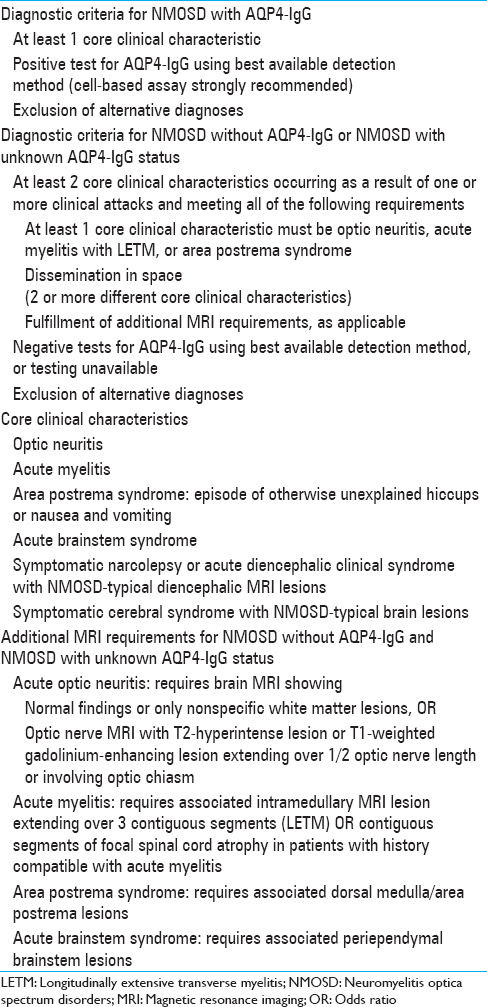
Table 3
International Panel for Neuromyelitis Optica Diagnosis Neuromyelitis Optica Spectrum Disorders Diagnostic Criteria for adult patients, Wingerchuk et al.[
Neuromyelitis optica spectrum disorder brain and optic nerve MRI
Paraclinical imaging of brain-associated MRI lesions is widely employed for NMOSD supportive diagnosis but most importantly as a tool for differentiating NMOSD from MS.[
In patients presenting with ON, T1-weighted gadolinium coronal images or an increase in T2-weighted MRI signal of optic nerve and optic chiasm are seen unilaterally or bilaterally, after 2 weeks of onset, and may be associated with severe vision loss.[
Neuromyelitis optica spectrum disorder optical coherence tomography
ON is one of the primary clinical manifestations detected in 55% of NMOSD patients and leads to progressive changes in vision, reduction in high or low contrast visual acuity, and potential complete vision loss within several weeks from disease onset.[
Neuromyelitis optica spectrum disorder spinal cord lesion MRI
The hallmark of NMOSD is acute myelitis, which is associated with acute continuous LETM lesion patterns that are detected via T2-weighted sagittal spinal MRI.[
Neuromyelitis optica spectrum disorder cerebrospinal fluid analysis
Examination of cerebrospinal fluid (CSF) can be performed in patients with acute NMOSD attacks since many NMOSD CSF distinctive cell types and biomarkers disappear once patients go into remission.[
Neuromyelitis optica spectrum disorder serum AQP4-AB IGG
Beyond certain characteristic clinical manifestations and specific neuroimaging findings, the presence of antibodies against water channel aquaporin-4 (AQP4), which is expressed in the astrocyte cell plasma membrane, is central for NMOSD diagnosis.[
TREATMENT OF NEUROMYELITIS OPTICA
Identifying an effective therapy for NMO is founded upon a basic understanding of the biological mechanisms that lead to the disease. Recent studies postulate that relapse in NMO disease has an association with chronic stress and hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis.[
The use of intravenous corticosteroids and/or plasmapheresis is two preventative measures to control sudden outbreaks for NMO patients. According to present literature, low-dose corticosteroid use is effective and reduces relapse rates in patients within a 1-year period.[
There are various options for treatment during remission on the market; however, selecting an appropriate treatment plan can be complicated. For this reason, choosing a first-line therapy is the focus of recent studies. Chemo-pharmaceuticals such as mycophenolate, cyclophosphamide, and methotrexate show certain therapeutic effects for plasma cell dyscrasia and immunosuppression.[
An alternative therapy is rituximab, a selective monoclonal antibody that reduces serum CD20+ B lymphocytes, with effects lasting weeks due to its long half-life which include observed benefits such as reduced relapse rates and maintained or improved patients’ neurological health.[
Therapy against key cytokines involved in NMO pathogenesis has drawn considerable interest. IL-6 is a key factor in B-cell differentiation and antibody production.[
Presently, interferon beta and natalizumab are no longer used as treatment options due to exacerbating NMO relapses or symptoms.[
DIETARY SUPPLEMENTATION IN NEUROMYELITIS OPTICA
Diet and nutrition, as either a management paradigm or as possible factor in NMO pathogenesis, has been minimally investigated. Current, cutting edge research, though, is starting to break the surface in three important areas: Vitamin D and its’ regulatory role in the immune system; cholesterol and cholesterol metabolites as inflammatory regulators; and finally, aberrations to the gut microbiome as inducers of pro-inflammatory T-helper cells.
Vitamin D
To date, there are no studies as to any palliative or curative efficacy of a vitamin D treatment in patients with NMO. MS research, though, has touched on the question and recent investigations into the effect of high-dose vitamin D therapies in reducing relapse rates are divided, some showing a promising reduction in relapse rates, while others are inconclusive.[
Cholesterol
For its role as a precursor in myelination of neurons by both CNS oligodendrocytes[
Gut microflora
One of the most interesting recent discoveries as to the actual pathogenesis of NMO may lie in the ubiquitous commensal flora of the human gut. Recent gut microbiome analyses of NMO patients show a marked overabundance C. perfringens.[
SYMPTOM MANAGEMENT OF NEUROMYELITIS OPTICA
NMO is a challenging neurodegenerative disease whose long-term management strategy is confounded by the high prevalence of pain, urinary incontinence, loss of mobility, fatigue, and depression.[
Mobility and ambulation
Physical therapy, in conjunction with pharmacological symptomatic management, is effective in improving measures of quality of life, functionality, and independence.[
Urinary function
A recent study focusing on urinary dysfunction in NMO patients found detrusor-sphincter dyssynergia and detrusor overactivity, either alone or together, in more than half of the patients assessed.[
Pain
With a prevalence of up to 86%, more than twice as common in occurrence compared to MS, and up to three times more severe, pain is a great challenge for management of NMO patients.[
Depression
A recent study showed a direct positive correlation between the severity of depression in NMO and the degree of neuropathic pain reported by patients.[
SURVIVAL AND PROGNOSIS OF NEUROMYELITIS OPTICA
The prognosis of NMO can be challenging. The disease has either a monophasic or relapsing nature. NMO relapse can serve as the main indicator for survival and prognosis.[
The age of onset of NMO can severely affect the patient's prognosis. The average age of onset for NMO is 39 years, but the disease can manifest in children and adults as well.[
The most important factor for survival and prognosis in NMO is relapse.[
Over 60% of patients will develop severe vision loss within the first two years of onset and a severe ambulatory disability within the first 5 years of the disease.[
Gender can affect the severity of NMO prognosis. Males have a higher age of onset (48.7 vs. 41 years, P = 0.037), and though they show a higher incidence rate of isolated myelitis, males present with a milder degree of ON upon onset and are more likely to not suffer ON attacks.[
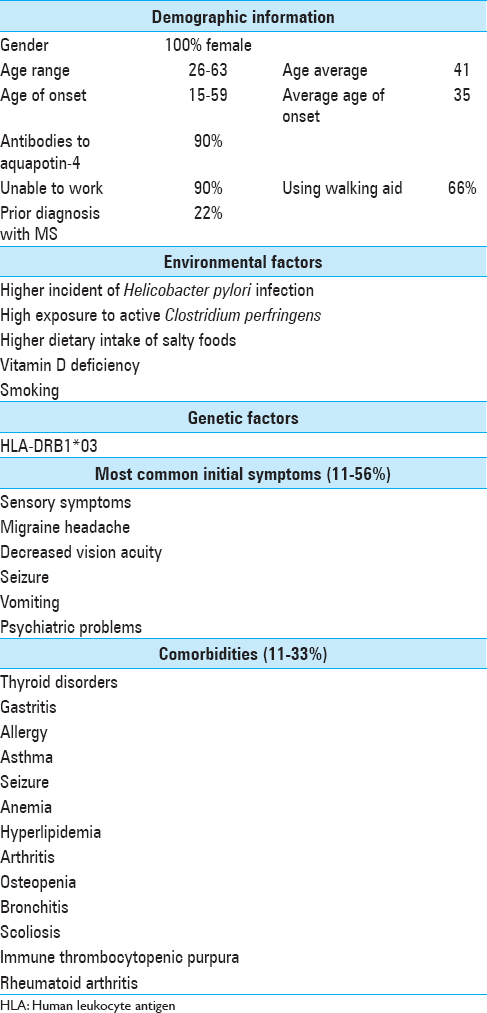
CHARACTERISTICS OF PATIENTS IN PUERTO RICO WITH NEUROMYELITIS OPTICA
Introduction
Despite recent advancements in understanding and diagnosing NMO, there has never been an epidemiological study of NMO patients from PR specifically.
For this study, we obtained the records of all NMO patients who visited Caribbean Neurological Center in PR.
Environmental factors
Gastritis is one of the unique comorbidities found in Puerto Rican NMO patients. Multiple studies have found higher incidence and prevalence of stomach cancer in PR compared to non-Hispanic Whites populations in the United States.[
Whether NMO patients in PR have active C. perfringens bacteria or not is not captured in our data. However, in a study done by Pait et al., chemical and biological contaminants in the marine sediments of southwest of PR were recorded and analyzed. According to their result, the southwest region of PR, specifically Guanica Bay, has over 1700 colony forming units of C. perfringes per gram of sediment sample.[
Despite conflicting evidence between populations, vitamin D deficiency could also be a risk factor for NMO illness. The population of PR is vitamin D deficient, with only 31.5% of the studied population having sufficient vitamin D levels.[
Smoking has been found as a most consistent nongenetic factor in different neurodegenerative diseases.[
Genetic factors
HLA-DRB1*03 was also the most prevalent allele found in MS patients from PR.[
Symptoms
Sensory symptoms, loss of motor strength in extremities, migraine headache, decreased vision acuity, seizure, vomiting, and psychiatric problems are among the most common initial symptoms in NMO patients from PR. Two of the nine patients were previously diagnosed with progressive-relapsing multiple sclerosis before being diagnosed with NMO due to similarities of symptoms between the two disorders.
Comorbidities
Thyroid abnormalities were the most frequent comorbidities among NMO patients in PR. Two of the common autoimmune disorders among Puerto Rican NMO patients were immune thrombocytopenic purpura and rheumatoid arthritis.
In a study conducted by Ajmera et al., they found comorbidities among NMO patients in United States that were reported in ≥2% of patients.[
Future studies measuring these factors in a broader range of NMO participants will be required to validate and to expand upon these results.
Survival and prognosis
The average onset age for Puerto Rican NMO patients was 35 ranging from 15 to 59 years old. The patient with older age of onset had more sever comorbidities compared to the younger ones. These included fibrocystic disease of breast, esophageal hiatus hernia, osteopenia, and osteoporosis. Whether these differences are as the result of late age of NMO onset or normal aging comorbidities needs to be further investigated.
More than half of Puerto Rican NMO patients had at least one relapse in 1-year period, which again is the most important factor for survival and prognosis. Long-term studies can chart these outcomes and capture prognostic data for future generations.
DISCUSSION
Even though according to the information we obtained that all the NMO patients from Caribbean Neurological Center were female, such a small prevalence of NMO and the lack of male patients suggest that a systemic collection and publication of NMO patients data and epidemiology could benefit the process of investigation, treatment innovation, and improvement of NMO within this population and worldwide.
CONCLUSION
Improving knowledge of NMO pathogenesis is critical in developing diagnostic methods for earlier detection and planning new effective treatments. Thus, an extensive understanding in a range of information regarding NMO epidemiology, environmental and genetic factors, molecular mechanism, symptoms, clinical differentiation, treatment, dietary supplementation, management, and survival and prognosis is essential. As of now, there is no treatment that completely cures the NMO and patients can face debilitating disabilities throughout the course of their disease. Multiple compound therapy including corticosteroid, immunosuppressant, monoclonal antibody, and plasmapheresis reduces the relapse rates and also decreases anti-AQP4 antibody serum levels in some cases.[
Although the underlying mechanism of NMO has already been elucidated, implicating predominantly the existence of (AQP4-IgG) antibodies in NMO patients, changes in ethnicity and environmental factors also show their pivotal role in variations of NMO clinical features and prognosis.
One of the main limitations of this study was our small sample size of NMO patients. This prevented us from performing any statistical analysis or hypothesis testing to establish comprehensive results. After reviewing all these patients’ information, our findings suggest a unique set of comorbidities among NMO patients in PR that were different compared to the NMO patients in United States. They also had higher female-to-male ratio with a wide range of age of onset from 15 to 59 years.
These differences could be associated with environmental and/or genetic factors that we discussed earlier in our study. They include higher incidents of gastritis, presence of HLA-DRB1 * 03 allele, higher exposure to C. perfringens, vitamin D deficiency, and higher daily intake of salt in diet. Some of these factors may be eliminated by reducing the exposures to the infectious agents and improvements in access to preventive and treatment care. Further research studies are certainly warranted to confirm the prevalence and epidemiology of NMO in PR and to understand the observed differences.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abkur TM, Foran E, Kearney H, Harkin G, Byrnes V, Lynch J. Neuromyelitis optica presenting as intractable vomiting and hyperCKaemia. J Neurol. 2016. 263: 171-3
2. Aiyer R, Mehta N, Gungor S, Gulati A. A systematic review of NMDA receptor antagonists for treatment of neuropathic pain in clinical practice. Clin J Pain. 2018. 34: 450-67
3. Ajmera MR, Boscoe A, Mauskopf J, Candrilli SD, Levy M. Evaluation of comorbidities and health care resource use among patients with highly active neuromyelitis optica. J Neurol Sci. 2018. 384: 96-103
4. Akaishi T, Takahashi T, Nakashima I. Chloride imbalance between serum and CSF in the acute phase of neuromyelitis optica. J Neuroimmunol. 2018. 315: 45-9
5. Akman-Demir G, Serdaroglu P, Tasçi B. Clinical patterns of neurological involvement in Behçet's disease: Evaluation of 200 patients. The neuro-Behçet study group. Brain. 1999. 122: 2171-82
6. Al Sawaf A, Berger JR. Longitudinally extensive transverse myelitis suspected for isolated neuro-Behçet: A diagnostic conundrum. Mult Scler Relat Disord. 2015. 4: 395-9
7. Al-Araji A, Kidd DP. Neuro-Behçet's disease: Epidemiology, clinical characteristics, and management. Lancet Neurol. 2009. 8: 192-204
8. Alharbi FM. Update in Vitamin D and multiple sclerosis. Neurosciences (Riyadh). 2015. 20: 329-35
9. Allen AC, Kelly S, Basdeo SA, Kinsella K, Mulready KJ, Mills KH. Apilot study of the immunological effects of high-dose Vitamin D in healthy volunteers. Mult Scler. 2012. 18: 1797-800
10. Alvarenga MP, Schimidt S, Alvarenga RP. Epidemiology of neuromyelitis optica in Latin America. Mult Scler J Exp Transl Clin. 2017. 3: 2055217317730098-
11. Amaral DM, Parreira T, Sampaio M. Longitudinally extensive transverse myelitis and meningitis due to a rare infectious cause. 2015. p. pii: Bcr2015211761-
12. Amirjamshidi A, Abbassioun K, Parsa K. Hiccup and neurosurgeons: A report of 4 rare dorsal medullary compressive pathologies and review of the literature. Surg Neurol. 2007. 67: 395-402
13. Anadure R, Narayanan CS, Varadraj G. Recurrent longitudinally extensive myelitis and aquaporin-4 seronegativity – The expanding spectrum of neuromyelitis optica. J Clin Diagn Res. 2017. 11: OD05-7
14. Apiwattanakul M, Popescu BF, Matiello M, Weinshenker BG, Lucchinetti CF, Lennon VA. Intractable vomiting as the initial presentation of neuromyelitis optica. Ann Neurol. 2010. 68: 757-61
15. Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: A pilot study. Neurology. 2014. 82: 1302-6
16. Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO. Apopulation-based study of neuromyelitis optica in Caucasians. Neurology. 2011. 76: 1589-95
17. Asgari N, Nielsen C, Stenager E, Kyvik KO, Lillevang ST. HLA, PTPN22 and PD-1 associations as markers of autoimmunity in neuromyelitis optica. Mult Scler. 2012. 18: 23-30
18. Ashtari F, Safaei A, Shaygannejad V, Najafi MA, Vesal S. Neuromyelitis optica spectrum disease characteristics in Isfahan, Iran: A cross-sectional study. J Res Med Sci. 2017. 22: 41-
19. Awad SA, Wilson JW, Fenemore J, Kiruluta HG. Dysfunction of the detrusor and urethra in multiple sclerosis: The role of drug therapy. Can J Surg. 1982. 25: 259-62
20. Azizlerli G, Köse AA, Sarica R, Gül A, Tutkun IT, Kulaç M. Prevalence of Behçet's disease in Istanbul, Turkey. Int J Dermatol. 2003. 42: 803-6
21. Baba T, Nakashima I, Kanbayashi T, Konno M, Takahashi T, Fujihara K. Narcolepsy as an initial manifestation of neuromyelitis optica with anti-aquaporin-4 antibody. J Neurol. 2009. 256: 287-8
22. Baehring JM, Hochberg FH, Betensky RA, Longtine J, Sklar J. Immunoglobulin gene rearrangement analysis in cerebrospinal fluid of patients with lymphoproliferative processes. J Neurol Sci. 2006. 247: 208-16
23. Baker RW, Thompson RH, Zilkha KJ. Serum fatty acids in multiple sclerosis subject age (yr.) diagnosis. J Neurol Neurosurg Psychiat. 1964. 27: 5-
24. Bandettini di Poggio M, Primavera A, Capello E, Bandini F, Mazzarello G, Viscoli C. Acase of secondary syphilis presenting as optic neuritis. Neurol Sci. 2010. 31: 365-7
25. Barbieri F, Buscaino GA. Neuromyelitis optica in the elderly. Acta Neurol (Napoli). 1989. 11: 247-51
26. Barros PO, Cassano T, Hygino J, Ferreira TB, Centurião N, Kasahara TM. Prediction of disease severity in neuromyelitis optica by the levels of interleukin (IL)-6 produced during remission phase. Clin Exp Immunol. 2016. 183: 480-9
27. Ben-Ari ET. Dual purpose: Some cancer therapies used to treat autoimmune diseases. J Natl Cancer Inst. 2004. 96: 577-9
28. Berghoff SA, Gerndt N, Winchenbach J, Stumpf SK, Hosang L, Odoardi F. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat Commun. 2017. 8: 14241-
29. Bichuetti DB, Lobato de Oliveira EM, Oliveira DM, Amorin de Souza N, Gabbai AA. Neuromyelitis optica treatment: Analysis of 36 patients. Arch Neurol. 2010. 67: 1131-6
30. Blanc F, Noblet V, Jung B, Rousseau F, Renard F, Bourre B. White matter atrophy and cognitive dysfunctions in neuromyelitis optica. PLoS One. 2012. 7: e33878-
31. Boström I, Stawiarz L, Landtblom AM. Sex ratio of multiple sclerosis in the national swedish MS register (SMSreg). Mult Scler. 2013. 19: 46-52
32. Bouzar M, Daoudi S, Hattab S, Bouzar AA, Deiva K, Wildemann B. Neuromyelitis optica spectrum disorders with antibodies to myelin oligodendrocyte glycoprotein or aquaporin-4: Clinical and paraclinical characteristics in Algerian patients. J Neurol Sci. 2017. 381: 240-4
33. Bradl M, Kanamori Y, Nakashima I, Misu T, Fujihara K, Lassmann H. Pain in neuromyelitis optica – Prevalence, pathogenesis and therapy. Nat Rev Neurol. 2014. 10: 529-36
34. Brum DG, Barreira AA, dos Santos AC, Kaimen-Maciel DR, Matiello M, Costa RM. HLA-DRB association in neuromyelitis optica is different from that observed in multiple sclerosis. Mult Scler. 2010. 16: 21-9
35. Burks JS, Bigley GK, Hill HH. Rehabilitation challenges in multiple sclerosis. Ann Indian Acad Neurol. 2009. 12: 296-306
36. Buser N, Ivic S, Kessler TM, Kessels AG, Bachmann LM. Efficacy and adverse events of antimuscarinics for treating overactive bladder: Network meta-analyses. Eur Urol. 2012. 62: 1040-60
37. Cabrera-Gómez JA, Kurtzke JF, González-Quevedo A, Lara-Rodríguez R. An epidemiological study of neuromyelitis optica in Cuba. J Neurol. 2009. 256: 35-44
38. Cai G, He D, Chu L, Dai Q, Xu Z, Zhang Y. Paraneoplastic neuromyelitis optica spectrum disorders: Three new cases and a review of the literature. Int J Neurosci. 2016. 126: 660-8
39. Calamia KT, Wilson FC, Icen M, Crowson CS, Gabriel SE, Kremers HM. Epidemiology and clinical characteristics of Behçet's disease in the US: A population-based study. Arthritis Rheum. 2009. 61: 600-4
40. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004. 229: 1136-42
41. Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015. 7: 3011-21
42. Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018. 359: 684-8
43. Carnero Contentti E, Daccach Marques V, Soto de Castillo I, Tkachuk V, Antunes Barreira A, Armas E. Frequency of brain MRI abnormalities in neuromyelitis optica spectrum disorder at presentation: A cohort of Latin American patients. Mult Scler Relat Disord. 2018. 19: 73-8
44. Cha E, Lee KM, Park KD, Park KS, Lee KW, Kim SM. Hydroxycholesterol levels in the serum and cerebrospinal fluid of patients with neuromyelitis optica revealed by LC-ag+CIS/MS/MS and LC-ESI/MS/MS with picolinic derivatization: Increased levels and association with disability during acute attack. PLoS One. 2016. 11: e0167819-
45. Chavarro VS, Mealy MA, Simpson A, Lacheta A, Pache F, Ruprecht K. Insufficient treatment of severe depression in neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflamm. 2016. 3: e286-
46. Chitnis T, Ness J, Krupp L, Waubant E, Hunt T, Olsen CS. Clinical features of neuromyelitis optica in children: US network of pediatric MS centers report. Neurology. 2016. 86: 245-52
47. Collongues N, de Seze J. Current and future treatment approaches for neuromyelitis optica. Ther Adv Neurol Disord. 2011. 4: 111-21
48. Collongues N, Marignier R, Zéphir H, Papeix C, Blanc F, Ritleng C. Neuromyelitis optica in France: A multicenter study of 125 patients. Neurology. 2010. 74: 736-42
49. Combes AJE, Matthews L, Lee JS, Li DKB, Carruthers R, Traboulsee AL. Cervical cord myelin water imaging shows degenerative changes over one year in multiple sclerosis but not neuromyelitis optica spectrum disorder. Neuroimage Clin. 2017. 16: 17-22
50. Corcos J. A urological challenge: Voiding dysfunction in multiple sclerosis. Can Urol Assoc J. 2013. 7: S181-2
51. Cornelio DB, Braga RP, Rosa MW, Ayub AC. Devic's neuromyelitis optica and pregnancy: Distinction from multiple sclerosis is essential. Arch Gynecol Obstet. 2009. 280: 475-7
52. Cossburn M, Tackley G, Baker K, Ingram G, Burtonwood M, Malik G. The prevalence of neuromyelitis optica in South East wales. Eur J Neurol. 2012. 19: 655-9
53. Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J. Azathioprine: Tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011. 77: 659-66
54. Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology. 2005. 64: 1270-2
55. Cree BA, Spencer CM, Varrin-Doyer M, Baranzini SE, Zamvil SS. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann Neurol. 2016. 80: 443-7
56. . International study group for Behçet's disease. Lancet. 1990. 335: 1078-80
57. Cruz-Herranz A, Sagan SA, Sobel RA, Green AJ, Zamvil SS. T cells targeting neuromyelitis optica autoantigen aquaporin-4 cause paralysis and visual system injury. J Nat Sci. 2017. 3: pii: e358-
58. Daoudi S, Bouzar M. Neuromyelitis optica spectrum disorders in Algeria: A preliminary study in the region of Tizi Ouzou. Mult Scler Relat Disord. 2016. 6: 37-40
59. de Bie J, Lim CK, Guillemin GJ. Progesterone alters kynurenine pathway activation in IFN-γ-activated macrophages – Relevance for neuroinflammatory diseases. Int J Tryptophan Res. 2016. 9: 89-93
60. de Carvalho FL, Gomes CM, Apostolos-Pereira SL, Bessa J, Pinheiro M, Marchiori PE. Voiding dysfunction in patients with neuromyelitis optica spectrum disorders. Neurourol Urodyn. 2016. 35: 39-43
61. de Oliveira P, de Carvalho DR, Brandi IV, Pratesi R. Serological prevalence of celiac disease in Brazilian population of multiple sclerosis, neuromyelitis optica and myelitis. Mult Scler Relat Disord. 2016. 9: 125-8
62. de Seze J, Lebrun C, Stojkovic T, Ferriby D, Chatel M, Vermersch P. Is Devic's neuromyelitis optica a separate disease. A comparative study with multiple sclerosis?. Mult Scler. 2003. 9: 521-5
63. Derle E, Güneş HN, Konuşkan B, Tuncer-Kurne A. Neuromyelitis optica in children: A review of the literature. Turk J Pediatr. 2014. 56: 573-80
64. Deschamps R, Paturel L, Jeannin S, Chausson N, Olindo S, Béra O. Different HLA class II (DRB1 and DQB1) alleles determine either susceptibility or resistance to NMO and multiple sclerosis among the French Afro-Caribbean population. Mult Scler. 2011. 17: 24-31
65. Dilokthornsakul P, Valuck RJ, Nair KV, Corboy JR, Allen RR, Campbell JD. Multiple sclerosis prevalence in the united states commercially insured population. Neurology. 2016. 86: 1014-21
66. Dmitrieva-Zdorova EV, Gabaeva MV, Seregin YA, Bodoev NV, Voronko OE. PDCD1 PD-1.3 polymorphism and allergic bronchial asthma in Russian and buryat patients. J Asthma. 2017. 54: 46-52
67. Downer JJ, Leite MI, Carter R, Palace J, Küker W, Quaghebeur G. Diagnosis of neuromyelitis optica (NMO) spectrum disorders: Is MRI obsolete?. Neuroradiology. 2012. 54: 279-85
68. Drori T, Chapman J. Diagnosis and classification of neuromyelitis optica (Devic's syndrome). Autoimmun Rev. 2014. 13: 531-3
69. Eaneff S, Wang V, Hanger M, Levy M, Mealy MA, Brandt AU. Patient perspectives on neuromyelitis optica spectrum disorders: Data from the patientsLikeMe online community. Mult Scler Relat Disord. 2017. 17: 116-22
70. Eskandarieh S, Nedjat S, Abdollahpour I, Moghadasi AN, Azimi AR, Sahraian MA. Comparing epidemiology and baseline characteristic of multiple sclerosis and neuromyelitis optica: A case-control study. Mult Scler Relat Disord. 2017. 12: 39-43
71. Etemadifar M, Nasr Z, Khalili B, Taherioun M, Vosoughi R. Epidemiology of neuromyelitis optica in the world: A systematic review and meta-analysis. Mult Scler Int 2015. 2015. p. 174720-
72. Felix CM, Levin MH, Verkman AS. Complement-independent retinal pathology produced by intravitreal injection of neuromyelitis optica immunoglobulin G. J Neuroinflammation. 2016. 13: 275-
73. Flanagan EP, Kaufmann TJ, Krecke KN, Aksamit AJ, Pittock SJ, Keegan BM. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol. 2016. 79: 437-47
74. Flanagan EP, O’Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011. 77: 784-91
75. Fragoso YD, Adoni T, Bichuetti DB, Brooks JB, Ferreira ML, Oliveira EM. Neuromyelitis optica and pregnancy. J Neurol. 2013. 260: 2614-9
76. Freitas E, Guimarães J. Neuromyelitis optica spectrum disorders associated with other autoimmune diseases. Rheumatol Int. 2015. 35: 243-53
77. Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995. 108: 2993-3002
78. Fu Q, Goodrum JF, Hayes C, Hostettler JD, Toews AD, Morell P. Control of cholesterol biosynthesis in Schwann cells. J Neurochem. 1998. 71: 549-55
79. Gajofatto A, Monaco S, Fiorini M, Zanusso G, Vedovello M, Rossi F. Assessment of outcome predictors in first-episode acute myelitis: A retrospective study of 53 cases. Arch Neurol. 2010. 67: 724-30
80. Gebhardt A, Buehler R, Wiest R, Tewald F, Sellner J, Humpert S. Mycoplasma pneumonia as a cause of neuromyelitis optica?. J Neurol. 2008. 255: 1268-9
81. Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation. 2008. 15: 251-9
82. Graves J, Grandhe S, Weinfurtner K, Krupp L, Belman A, Chitnis T. Protective environmental factors for neuromyelitis optica. Neurology. 2014. 83: 1923-9
83. Halkjær SI, Boolsen AW, Günther S, Christensen AH, Petersen AM. Can fecal microbiota transplantation cure irritable bowel syndrome?. World J Gastroenterol. 2017. 23: 4112-20
84. Hall LM, Kimlin MG, Aronov PA, Hammock BD, Slusser JR, Woodhouse LR. Vitamin D intake needed to maintain target serum 25-hydroxyvitamin D concentrations in participants with low sun exposure and dark skin pigmentation is substantially higher than current recommendations. J Nutr. 2010. 140: 542-50
85. He D, Chen X, Zhao D, Zhou H. Cognitive function, depression, fatigue, and activities of daily living in patients with neuromyelitis optica after acute relapse. Int J Neurosci. 2011. 121: 677-83
86. He D, Wu Q, Chen X, Zhao D, Gong Q, Zhou H. Cognitive impairment and whole brain diffusion in patients with neuromyelitis optica after acute relapse. Brain Cogn. 2011. 77: 80-8
87. Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of graves’ disease: Evidence of a genetic and an environmental contribution. J Autoimmun. 2010. 34: J307-13
88. Hinson SR, Roemer SF, Lucchinetti CF, Fryer JP, Kryzer TJ, Chamberlain JL. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med. 2008. 205: 2473-81
89. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European association for neuro-oncology. Lancet Oncol. 2015. 16: e322-32
90. Houzen H, Niino M, Hirotani M, Fukazawa T, Kikuchi S, Tanaka K. Increased prevalence, incidence, and female predominance of multiple sclerosis in Northern Japan. J Neurol Sci. 2012. 323: 117-22
91. Hsu CL, Yeh JH, Lau CI. Persistent hyperthermia in a patient with aquaporin-4-antibody-positive neuromyelitis optica spectrum disorder. J Clin Neurol. 2016. 12: 515-6
92. Huppke P, Blüthner M, Bauer O, Stark W, Reinhardt K, Huppke B. Neuromyelitis optica and NMO-IgG in European pediatric patients. Neurology. 2010. 75: 1740-4
93. Hurst RW, Kenyon LC, Lavi E, Raps EC, Marcotte P. Spinal dural arteriovenous fistula: The pathology of venous hypertensive myelopathy. Neurology. 1995. 45: 1309-13
94. Hyun JW, Jeong IH, Joung A, Kim SH, Kim HJ. Evaluation of the 2015 diagnostic criteria for neuromyelitis optica spectrum disorder. Neurology. 2016. 86: 1772-9
95. Iorio R, Lucchinetti CF, Lennon VA, Farrugia G, Pasricha PJ, Weinshenker BG. Intractable nausea and vomiting from autoantibodies against a brain water channel. Clin Gastroenterol Hepatol. 2013. 11: 240-5
96. Isobe N, Oksenberg JR. Genetic studies of multiple sclerosis and neuromyelitis optica: Current status in European, African American and Asian populations. Clin Exp Neuroimmunol. 2014. 5: 61-8
97. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009. 139: 485-98
98. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+T helper cells. Cell. 2006. 126: 1121-33
99. Jacob A, Matiello M, Wingerchuk DM, Lucchinetti CF, Pittock SJ, Weinshenker BG. Neuromyelitis optica: Changing concepts. J Neuroimmunol. 2007. 187: 126-38
100. Jain RS, Kumar S, Tejwani S. A rare association of tuberculous longitudinally extensive transverse myelitis (LETM) with brain tuberculoma. Springerplus. 2015. 4: 476-
101. Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J Gastroenterol. 2006. 12: 4296-303
102. James E, Dobson R, Kuhle J, Baker D, Giovannoni G, Ramagopalan SV. The effect of Vitamin D-related interventions on multiple sclerosis relapses: A meta-analysis. Mult Scler. 2013. 19: 1571-9
103. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996. 383: 728-31
104. Jarius S, Jacobi C, de Seze J, Zephir H, Paul F, Franciotta D. Frequency and syndrome specificity of antibodies to aquaporin-4 in neurological patients with rheumatic disorders. Mult Scler. 2011. 17: 1067-73
105. Jarius S, Paul F, Franciotta D, de Seze J, Münch C, Salvetti M. Neuromyelitis optica spectrum disorders in patients with myasthenia gravis: Ten new aquaporin-4 antibody positive cases and a review of the literature. Mult Scler. 2012. 18: 1135-43
106. Jarius S, Paul F, Franciotta D, Ruprecht K, Ringelstein M, Bergamaschi R. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: Results from 211 lumbar punctures. J Neurol Sci. 2011. 306: 82-90
107. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K. MOG-igG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016. 13: 280-
108. Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation. 2012. 9: 14-
109. Jarius S, Wildemann B, Paul F. Neuromyelitis optica: Clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014. 176: 149-64
110. Jarius S, Wildemann B. The history of neuromyelitis optica. J Neuroinflammation. 2013. 10: 8-
111. Jellema K, Canta LR, Tijssen CC, van Rooij WJ, Koudstaal PJ, van Gijn J. Spinal dural arteriovenous fistulas: Clinical features in 80 patients. J Neurol Neurosurg Psychiatry. 2003. 74: 1438-40
112. Jin X, Pei S, Liu Y, Li X. Clinical analysis of neuromyelitis optica presenting as intractable nausea, vomiting and hiccups. Int J Neurosci. 2017. 127: 854-8
113. Jitprapaikulsan J, Siritho S, Prayoonwiwat N. Vitamin D level status in thai neuromyelitis optica patients. J Neuroimmunol. 2016. 295-296: 75-8
114. Jitprapaikulsan SS. Vitamin D and neuromyelitis optica spectrum disorders. Austin J Mult Scler Neuroimmunol. 2016. 3: 1-3
115. Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: Comparison with histopathologic features. AJNR Am J Neuroradiol. 1997. 18: 563-72
116. Juryńczyk M, Craner M, Palace J. Overlapping CNS inflammatory diseases: Differentiating features of NMO and MS. J Neurol Neurosurg Psychiatry. 2015. 86: 20-5
117. Kagawa Y. Impact of westernization on the nutrition of Japanese: Changes in physique, cancer, longevity and centenarians. Prev Med. 1978. 7: 205-17
118. Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS. Diagnosis and management of neuro-Behçet's disease: International consensus recommendations. J Neurol. 2014. 261: 1662-76
119. Kalsi V, Fowler CJ. Therapy insight: Bladder dysfunction associated with multiple sclerosis. Nat Clin Pract Urol. 2005. 2: 492-501
120. Kampman MT, Steffensen LH. The role of Vitamin D in multiple sclerosis. J Photochem Photobiol B. 2010. 101: 137-41
121. Kanamori Y, Nakashima I, Takai Y, Nishiyama S, Kuroda H, Takahashi T. Pain in neuromyelitis optica and its effect on quality of life: A cross-sectional study. Neurology. 2011. 77: 652-8
122. Kanbayashi T, Shimohata T, Nakashima I, Yaguchi H, Yabe I, Nishizawa M. Symptomatic narcolepsy in patients with neuromyelitis optica and multiple sclerosis: New neurochemical and immunological implications. Arch Neurol. 2009. 66: 1563-6
123. Kanoto M, Hosoya T, Toyoguchi Y, Oda A. Brain stem and cerebellar atrophy in chronic progressive neuro-Behçet's disease. Eur J Radiol. 2013. 82: 146-50
124. Kessler RA, Mealy MA, Jimenez-Arango JA, Quan C, Paul F, López R. Anti-aquaporin-4 titer is not predictive of disease course in neuromyelitis optica spectrum disorder: A multicenter cohort study. Mult Scler Relat Disord. 2017. 17: 198-201
125. Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: Acute, preventive, and symptomatic. Curr Treat Options Neurol. 2016. 18: 2-
126. Kikuchi H, Takayama M, Hirohata S. Quantitative analysis of brainstem atrophy on magnetic resonance imaging in chronic progressive neuro-Behçet's disease. J Neurol Sci. 2014. 337: 80-5
127. Kim HJ, Park HY, Kim E, Lee KS, Kim KK, Choi BO. Common CYP7A1 promoter polymorphism associated with risk of neuromyelitis optica. Neurobiol Dis. 2010. 37: 349-55
128. Kim SM, Go MJ, Sung JJ, Park KS, Lee KW. Painful tonic spasm in neuromyelitis optica: Incidence, diagnostic utility, and clinical characteristics. Arch Neurol. 2012. 69: 1026-31
129. Kim SM, Kim SJ, Lee HJ, Kuroda H, Palace J, Fujihara K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther Adv Neurol Disord. 2017. 10: 265-89
130. Kim SM, Waters P, Vincent A, Kim SY, Kim HJ, Hong YH. Sjogren's syndrome myelopathy: Spinal cord involvement in Sjogren's syndrome might be a manifestation of neuromyelitis optica. Mult Scler. 2009. 15: 1062-8
131. Kim SM, Waters P, Woodhall M, Kim JY, Kim JE, Yang JW. Utility of aquaporin-4 antibody assay in patients with neuromyelitis optica spectrum disorders. Mult Scler. 2013. 19: 1060-7
132. Kim SM, Waters P, Woodhall M, Yang JW, Yang H, Kim JE. Characterization of the spectrum of Korean inflammatory demyelinating diseases according to the diagnostic criteria and AQP4-ab status. BMC Neurol. 2014. 14: 93-
133. Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A. Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2015. 2: e163-
134. Kimbrough DJ, Fujihara K, Jacob A, Lana-Peixoto MA, Leite MI, Levy M. Treatment of neuromyelitis optica: Review and recommendations. Mult Scler Relat Disord. 2012. 1: 180-7
135. Kinoshita M, Obata K, Tanaka M. Latitude has more significant impact on prevalence of multiple sclerosis than ultraviolet level or Sunshine duration in Japanese population. Neurol Sci. 2015. 36: 1147-51
136. Kira J. Genetic and environmental factors underlying the rapid changes in epidemiological and clinical features of multiple sclerosis and neuromyelitis optica in Japanese. Clin Exp Neuroimmunol. 2013. 4: 261-73
137. Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int 2013. 2013. p.
138. Kitley J, Elsone L, George J, Waters P, Woodhall M, Vincent A. Methotrexate is an alternative to azathioprine in neuromyelitis optica spectrum disorders with aquaporin-4 antibodies. J Neurol Neurosurg Psychiatry. 2013. 84: 918-21
139. Kitley J, Waters P, Woodhall M, Leite MI, Murchison A, George J. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: A comparative study. JAMA Neurol. 2014. 71: 276-83
140. Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013. 496: 518-22
141. Kleiter I, Hellwig K, Berthele A, Kümpfel T, Linker RA, Hartung HP. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012. 69: 239-45
142. Koelman DL, Chahin S, Mar SS, Venkatesan A, Hoganson GM, Yeshokumar AK. Acute disseminated encephalomyelitis in 228 patients: A retrospective, multicenter US study. Neurology. 2016. 86: 2085-93
143. Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult Scler. 2013. 19: 1261-7
144. Kumar N, Keegan BM, Rodriguez FJ, Hammack JE, Kantarci OH. Intravascular lymphoma presenting as a longitudinally-extensive myelitis: Diagnostic challenges and etiologic clues. J Neurol Sci. 2011. 303: 146-9
145. Kume K, Deguchi K, Ikeda K, Takata T, Kokudo Y, Kamada M. Neuromyelitis optica spectrum disorder presenting with repeated hypersomnia due to involvement of the hypothalamus and hypothalamus-amygdala linkage. Mult Scler. 2015. 21: 960-2
146. Lalan S, Khan M, Schlakman B, Penman A, Gatlin J, Herndon R. Differentiation of neuromyelitis optica from multiple sclerosis on spinal magnetic resonance imaging. Int J MS Care. 2012. 14: 209-14
147. Larik A, Chiong Y, Lee LC, Ng YS. Longitudinally extensive transverse myelitis associated with dengue fever. BMJ Case Rep 2012. 2012. p. pii: bcr1220115378-
148. Laursen JH, Søndergaard HB, Sørensen PS, Sellebjerg F, Oturai AB. Vitamin D supplementation reduces relapse rate in relapsing-remitting multiple sclerosis patients treated with natalizumab. Mult Scler Relat Disord. 2016. 10: 169-73
149. Lee HS, Kim do Y, Shin HY, Choi YC, Kim SM. Spinal cord involvement in Behçet's disease. Mult Scler. 2016. 22: 960-3
150. Lee JDLast accessed on 2018 Apr 09. Available from: https://www.open.library.ubc.ca/cIRcle/collections/ubctheses/24/items/1.0165922 .
151. Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011. 108: 4615-22
152. Leite MI, Coutinho E, Lana-Peixoto M, Apostolos S, Waters P, Sato D. Myasthenia gravis and neuromyelitis optica spectrum disorder: A multicenter study of 16 patients. Neurology. 2012. 78: 1601-7
153. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005. 202: 473-7
154. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet. 2004. 364: 2106-12
155. Li W, Minohara M, Piao H, Matsushita T, Masaki K, Matsuoka T. Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult Scler. 2009. 15: 1411-21
156. Liu J, Shi Z, Lian Z, Chen H, Zhang Q, Feng H. Association of CD58 gene polymorphisms with NMO spectrum disorders in a Han Chinese population. J Neuroimmunol. 2017. 309: 23-30
157. Long Y, Zheng Y, Chen M, Zhang B, Gao C, Shan F. Serum thyroid-stimulating hormone and anti-thyroglobulin antibody are independently associated with lesions in spinal cord in central nervous system demyelinating diseases. PLoS One. 2014. 9: e100672-
158. Lovera J, Reza T. Stress in multiple sclerosis: Review of new developments and future directions. Curr Neurol Neurosci Rep. 2013. 13: 398-
159. Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV. Sun exposure and Vitamin D are independent risk factors for CNS demyelination. Neurology. 2011. 76: 540-8
160. Luppe S, Robertson NP. MOG-IgG in neuromyelitis optica. J Neurol. 2014. 261: 640-2
161. Marignier R, De Sèze J, Vukusic S, Durand-Dubief F, Zéphir H, Vermersch P. NMO-igG and devic's neuromyelitis optica: A French experience. Mult Scler. 2008. 14: 440-5
162. Martínez-Lapiscina EH, Fraga-Pumar E, Pastor X, Gómez M, Conesa A, Lozano-Rubí R. Is the incidence of optic neuritis rising? Evidence from an epidemiological study in barcelona (Spain), 2008-2012. J Neurol. 2014. 261: 759-67
163. Masters-Israilov A, Robbins MS. Headache in neuromyelitis optica. Curr Pain Headache Rep. 2017. 21: 20-
164. Masuda H, Mori M, Uzawa A, Muto M, Uchida T, Kuwabara S. Serum antinuclear antibody may be associated with less severe disease activity in neuromyelitis optica. Eur J Neurol. 2016. 23: 276-81
165. Mateo J, Esteban O, Martínez M, Grzybowski A, Ascaso FJ. The contribution of optical coherence tomography in neuromyelitis optica spectrum disorders. Front Neurol. 2017. 8: 493-
166. Matiello M, Lennon VA, Jacob A, Pittock SJ, Lucchinetti CF, Wingerchuk DM. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008. 70: 2197-200
167. Matiello M, Schaefer-Klein JL, Hebrink DD, Kingsbury DJ, Atkinson EJ, Weinshenker BG. Genetic analysis of aquaporin-4 in neuromyelitis optica. Neurology. 2011. 77: 1149-55
168. Matthews L, Marasco R, Jenkinson M, Küker W, Luppe S, Leite MI. Distinction of seropositive NMO spectrum disorder and MS brain lesion distribution. Neurology. 2013. 80: 1330-7
169. McKeon A, Lennon VA, Jacob A, Matiello M, Lucchinetti CF, Kale N. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve. 2009. 39: 87-90
170. Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: A multicenter analysis. Arch Neurol. 2012. 69: 1176-80
171. Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: Multicenter study of treatment efficacy. JAMA Neurol. 2014. 71: 324-30
172. Mehnert U, Birzele J, Reuter K, Schurch B. The effect of botulinum toxin type a on overactive bladder symptoms in patients with multiple sclerosis: A pilot study. J Urol. 2010. 184: 1011-6
173. Merle H, Olindo S, Bonnan M, Donnio A, Richer R, Smadja D. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. 2007. 114: 810-5
174. Meurs L, Labeye D, Declercq I, Piéret F, Gille M. Acute transverse myelitis as a main manifestation of early stage II neuroborreliosis in two patients. Eur Neurol. 2004. 52: 186-8
175. Michel MC, Barendrecht MM. Physiological and pathological regulation of the autonomic control of urinary bladder contractility. Pharmacol Ther. 2008. 117: 297-312
176. Miclea A, Miclea M, Pistor M, Hoepner A, Chan A, Hoepner R. Vitamin D supplementation differentially affects seasonal multiple sclerosis disease activity. Brain Behav. 2017. 7: e00761-
177. Milo R, Kahana E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010. 9: A387-94
178. Min JH, Kim HJ, Kim BJ, Lee KW, Sunwoo IN, Kim SM. Brain abnormalities in Sjogren syndrome with recurrent CNS manifestations: Association with neuromyelitis optica. Mult Scler. 2009. 15: 1069-76
179. Min JH, Waters P, Vincent A, Cho HJ, Joo BE, Woo SY. Low levels of Vitamin D in neuromyelitis optica spectrum disorder: Association with disease disability. PLoS One. 2014. 9: e107274-
180. Miranda MT, Suárez E, Abbas M, Chinea A, Tosado R, Mejías IA. HLA class I & II alleles in multiple sclerosis patients from Puerto Rico. Bol Asoc Med P R. 2013. 105: 18-23
181. Mirsattari SM, Johnston JB, McKenna R, Del Bigio MR, Orr P, Ross RT. Aboriginals with multiple sclerosis: HLA types and predominance of neuromyelitis optica. Neurology. 2001. 56: 317-23
182. Miyamoto K, Fujihara K, Kira JI, Kuriyama N, Matsui M, Tamakoshi A. The prevalence and characteristics of neuromyelitis optica in Japan: A nationwide epidemiological study. J Neurol Sci. 2017. 381: 787-
183. Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human foxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011. 10: 744-55
184. Mok CC, To CH, Mak A, Poon WL. Immunoablative cyclophosphamide for refractory lupus-related neuromyelitis optica. J Rheumatol. 2008. 35: 172-4
185. Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. J Neuroimmune Pharmacol. 2012. 7: 42-59
186. Moore P, Methley A, Pollard C, Mutch K, Hamid S, Elsone L. Cognitive and psychiatric comorbidities in neuromyelitis optica. J Neurol Sci. 2016. 360: 4-9
187. Morrow MJ, Wingerchuk D. Neuromyelitis optica. J Neuroophthalmol. 2012. 32: 154-66
188. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006. 296: 2832-8
189. Muto M, Mori M, Sato Y, Uzawa A, Masuda S, Kuwabara S. Seasonality of multiple sclerosis and neuromyelitis optica exacerbations in Japan. Mult Scler. 2013. 19: 378-9
190. Nakajima H, Furutama D, Kimura F, Shinoda K, Ohsawa N, Nakagawa T. Herpes simplex virus myelitis: Clinical manifestations and diagnosis by the polymerase chain reaction method. Eur Neurol. 1998. 39: 163-7
191. Nakajima H, Hosokawa T, Sugino M, Kimura F, Sugasawa J, Hanafusa T. Visual field defects of optic neuritis in neuromyelitis optica compared with multiple sclerosis. BMC Neurol. 2010. 10: 45-
192. Nakamura H, Usa T, Motomura M, Ichikawa T, Nakao K, Kawasaki E. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest. 2008. 31: 861-5
193. Nakano H, Tanaka M, Kinoshita M, Tahara M, Matsui M, Tanaka K. Epileptic seizures in Japanese patients with multiple sclerosis and neuromyelitis optica. Epilepsy Res. 2013. 104: 175-80
194. Nazario CM, Szklo M, Diamond E, Román-Franco A, Climent C, Suarez E. Salt and gastric cancer: A case-control study in Puerto Rico. Int J Epidemiol. 1993. 22: 790-7
195. Nechemia Y, Moreh E, Weingarden H, Bloch A, Givon U, Vaknin-Dembinsky A. Effectiveness of multi-disciplinary rehabilitation for patients with neuromyelitis optica. J Spinal Cord Med. 2016. 39: 311-6
196. Nour MM, Nakashima I, Coutinho E, Woodhall M, Sousa F, Revis J. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology. 2016. 86: 79-87
197. O’Connell K, Marnane M, McGuigan C. Bilateral ocular perineuritis as the presenting feature of acute syphilis infection. J Neurol. 2012. 259: 191-2
198. Oertel FC, Zimmermann H, Paul F, Brandt AU. Optical coherence tomography in neuromyelitis optica spectrum disorders: Potential advantages for individualized monitoring of progression and therapy. EPMA J. 2018. 9: 21-33
199. Ogasawara M, Meguro A, Sakai T, Mizuki N, Takahashi T, Fujihara K. Genetic analysis of the aquaporin-4 gene for anti-AQP4 antibody-positive neuromyelitis optica in a Japanese population. Jpn J Ophthalmol. 2016. 60: 198-205
200. Ortiz AP, Soto-Salgado M, Calo WA, Tortolero-Luna G, Pérez CM, Romero CJ. Incidence and mortality rates of selected infection-related cancers in Puerto Rico and in the United States. Infect Agent Cancer. 2010. 5: 10-
201. Pait AS, Whitall DR, Jeffrey CF, Caldow C, Mason AL, Lauenstein GG. Chemical contamination in Southwest Puerto Rico: An assessment of organic contaminants in nearshore sediments. Mar Pollut Bull. 2008. 56: 580-7
202. Pamer EG. Fecal microbiota transplantation: Effectiveness, complexities, and lingering concerns. Mucosal Immunol. 2014. 7: 210-4
203. Pan J, Zhao P, Cai H, Su L, Wood K, Shi FD. Hypoxemia, sleep disturbances, and depression correlated with fatigue in neuromyelitis optica spectrum disorder. CNS Neurosci Ther. 2015. 21: 599-606
204. Pandit L, Kundapur R. Prevalence and patterns of demyelinating central nervous system disorders in urban Mangalore, South India. Mult Scler. 2014. 20: 1651-3
205. Pandit L, Mustafa S, Kunder R, Shetty R, Misri Z, Pai S. Optimizing the management of neuromyelitis optica and spectrum disorders in resource poor settings: Experience from the mangalore demyelinating disease registry. Ann Indian Acad Neurol. 2013. 16: 572-6
206. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol. 2012. 11: 535-44
207. Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013. 14: 265-77
208. Papais-Alvarenga RM, Miranda-Santos CM, Puccioni-Sohler M, de Almeida AM, Oliveira S, Basilio De Oliveira CA. Optic neuromyelitis syndrome in Brazilian patients. J Neurol Neurosurg Psychiatry. 2002. 73: 429-35
209. Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A, Stankoff B. Immunosuppressive therapy is more effective than interferon in neuromyelitis optica. Mult Scler. 2007. 13: 256-9
210. Parker SE, Pula JH. Neurosyphilis presenting as asymptomatic optic perineuritis. Case Rep Ophthalmol Med 2012. 2012. p.
211. Pellkofer HL, Havla J, Hauer D, Schelling G, Azad SC, Kuempfel T. The major brain endocannabinoid 2-AG controls neuropathic pain and mechanical hyperalgesia in patients with neuromyelitis optica. PLoS One. 2013. 8: e71500-
212. Pellkofer HL, Krumbholz M, Berthele A, Hemmer B, Gerdes LA, Havla J. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011. 76: 1310-5
213. Pereira CM, Castiglione M, Kasawara KT. Effects of physiotherapy treatment for urinary incontinence in patient with multiple sclerosis. J Phys Ther Sci. 2017. 29: 1259-63
214. Pereira WL, Reiche EM, Kallaur AP, Kaimen-Maciel DR. Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: A review. J Neurol Sci. 2015. 355: 7-17
215. Petajan JH, Gappmaier E, White AT, Spencer MK, Mino L, Hicks RW. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996. 39: 432-41
216. Petković F, Castellano B. The role of interleukin-6 in central nervous system demyelination. Neural Regen Res. 2016. 11: 1922-3
217. Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and New Latinos. Cancer Epidemiol Biomarkers Prev. 2009. 18: 2162-9
218. Pisani F, Mastrototaro M, Rossi A, Nicchia GP, Tortorella C, Ruggieri M. Identification of two major conformational aquaporin-4 epitopes for neuromyelitis optica autoantibody binding. J Biol Chem. 2011. 286: 9216-24
219. Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008. 65: 78-83
220. Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol. 2006. 63: 390-6
221. Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: An open-label pilot study. Lancet Neurol. 2013. 12: 554-62
222. Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol. 2006. 63: 964-8
223. Popescu BF, Lennon VA, Parisi JE, Howe CL, Weigand SD, Cabrera-Gómez JA. Neuromyelitis optica unique area postrema lesions: Nausea, vomiting, and pathogenic implications. Neurology. 2011. 76: 1229-37
224. Prokunina L, Padyukov L, Bennet A, de Faire U, Wiman B, Prince J. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum. 2004. 50: 1770-3
225. Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, Naismith RT. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol. 2012. 69: 1482-7
226. Rafael H. Omental transplantation against nmo and MS: A method to Decrease or Prevent Relapses. Neurol Clin Therapeut J. 2018. 2: 102-
227. Rafael H. Omental transplantation for neuromyelitis optica. J Neurol Stroke. 2017. 6: 00194-
228. Ramanathan S, Prelog K, Barnes EH, Tantsis EM, Reddel SW, Henderson AP. Radiological differentiation of optic neuritis with myelin oligodendrocyte glycoprotein antibodies, aquaporin-4 antibodies, and multiple sclerosis. Mult Scler. 2016. 22: 470-82
229. Ramírez-Vick M, Hernández-Dávila L, Rodríguez-Rivera N, López-Valentín M, Haddock L, Rodríguez-Martínez R. Prevalence of Vitamin D insufficiency and deficiency among young physicians at university district hospital in San Juan, Puerto Rico. P R Health Sci J. 2015. 34: 83-8
230. Rodríguez-Quiroga SA, Abaroa L, Arakaki T, Garretto NS, Villa AM. Commentary on neuromyelitis optica associated with painful paroxysmal dystonia: Case report and literature review. Acta Neurol Belg. 2015. 115: 523-4
231. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005. 65: 812-9
232. Rosito S, Nicchia GP, Palazzo C, Lia A, Buccoliero C, Pisani F. Supramolecular aggregation of aquaporin.4 is different in muscle and brain: Correlation with tissue susceptibility in neuromyelitis optica. J Cell Mol Med. 2018. 22: 1236-46
233. Rumah KR, Linden J, Fischetti VA, Vartanian T. Isolation of clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS One. 2013. 8: e76359-
234. Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997. 145: 234-41
235. Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa R, Wehr MC. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005. 8: 468-75
236. Sahraian MA, Moghadasi AN, Azimi AR, Asgari N, H Akhoundi F, Abolfazli R. Diagnosis and management of neuromyelitis optica spectrum disorder (NMOSD) in Iran: A consensus guideline and recommendations. Mult Scler Relat Disord. 2017. 18: 144-51
237. Sang CN. NMDA-receptor antagonists in neuropathic pain: Experimental methods to clinical trials. J Pain Symptom Manage. 2000. 19: S21-5
238. Sato DK, Callegaro D, Lana-Peixoto MA, Nakashima I, Fujihara K. Seronegative neuromyelitis optica spectrum – The challenges on disease definition and pathogenesis. Arq Neuropsiquiatr. 2014. 72: 445-50
239. Satoh J, Obayashi S, Misawa T, Tabunoki H, Yamamura T, Arima K. Neuromyelitis optica/Devic's disease: Gene expression profiling of brain lesions. Neuropathology. 2008. 28: 561-76
240. Schreiber AL, Fried GW, Formal CS, DeSouza BX. Rehabilitation of neuromyelitis optica (Devic syndrome): Three case reports. Am J Phys Med Rehabil. 2008. 87: 144-8
241. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013. 70: 311-9
242. Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010. 17: 1019-32
243. Seok JM, Cho HJ, Ahn SW, Cho EB, Park MS, Joo IS. Clinical characteristics of late-onset neuromyelitis optica spectrum disorder: A multicenter retrospective study in Korea. Mult Scler. 2017. 23: 1748-56
244. Sepúlveda M, Armangué T, Sola-Valls N, Arrambide G, Meca-Lallana JE, Oreja-Guevara C. Neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflammation. 2016. 3: e225-
245. Shan Y, Tan S, Zhang L, Huang J, Sun X, Wang Y. Serum 25-hydroxyvitamin D3 is associated with disease status in patients with neuromyelitis optica spectrum disorders in South China. J Neuroimmunol. 2016. 299: 118-23
246. Sheng P, Hou L, Wang X, Wang X, Huang C, Yu M. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: A systematic review and meta-analysis. PLoS One. 2013. 8: e81802-
247. Sherman E, Han MH. Acute and chronic management of neuromyelitis optica spectrum disorder. Curr Treat Options Neurol. 2015. 17: 48-
248. Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000. 18: 423-49
249. Simon KC, Schmidt H, Loud S, Ascherio A. Risk factors for multiple sclerosis, neuromyelitis optica and transverse myelitis. Mult Scler. 2015. 21: 703-9
250. Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E. Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian J Med Res. 2013. 138: 591-4
251. Singhrao SK, Neal JW, Rushmere NK, Morgan BP, Gasque P. Spontaneous classical pathway activation and deficiency of membrane regulators render human neurons susceptible to complement lysis. Am J Pathol. 2000. 157: 905-18
252. Smith GT, Goldmeier D, Migdal C. Neurosyphilis with optic neuritis: An update. Postgrad Med J. 2006. 82: 36-9
253. Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006. 8: 383-95
254. Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006. 177: 6030-7
255. Spooren A, Kooijman R, Lintermans B, Van Craenenbroeck K, Vermeulen L, Haegeman G. Cooperation of NFkappaB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal. 2010. 22: 871-81
256. Stern BJ, Krumholz A, Johns C, Scott P, Nissim J. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985. 42: 909-17
257. Stiebel-Kalish H, Lotan I, Brody J, Chodick G, Bialer O, Marignier R. Retinal nerve fiber layer may be better preserved in MOG-IgG versus AQP4-IgG optic neuritis: A Cohort study. PLoS One. 2017. 12: e0170847-
258. Storoni M, Petzold A, Plant GT. The use of serum glial fibrillary acidic protein measurements in the diagnosis of neuromyelitis optica spectrum optic neuritis. PLoS One. 2011. 6: e23489-
259. Suárez-Martínez EB, Pérez CM, Cruz SK, Khorsandi S, Chardón C, Ferder L. Importance of Vitamin D and Vitamin D levels status in Puerto Ricans. J Health Care Poor Underserved. 2013. 24: 38-47
260. Suzuki K, Nakamura T, Hashimoto K, Miyamoto M, Komagamine T, Nagashima T. Hypothermia, hypotension, hypersomnia, and obesity associated with hypothalamic lesions in a patient positive for the anti-aquaporin 4 antibody: A case report and literature review. Arch Neurol. 2012. 69: 1355-9
261. Takahashi T, Miyazawa I, Misu T, Takano R, Nakashima I, Fujihara K. Intractable hiccup and nausea in neuromyelitis optica with anti-aquaporin-4 antibody: A herald of acute exacerbations. J Neurol Neurosurg Psychiatry. 2008. 79: 1075-8
262. Tanaka M, Tanaka K. Sudden hearing loss as the initial symptom in Japanese patients with multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. J Neuroimmunol. 2016. 298: 16-8
263. Tanaka T, Kishimoto T. Targeting interleukin-6: All the way to treat autoimmune and inflammatory diseases. Int J Biol Sci. 2012. 8: 1227-36
264. Tenembaum S, Chitnis T, Nakashima I, Collongues N, McKeon A, Levy M. Neuromyelitis optica spectrum disorders in children and adolescents. Neurology. 2016. 87: S59-66
265. Thorlacius S, Aarli JA, Riise T, Matre R, Johnsen HJ. Associated disorders in myasthenia gravis: Autoimmune diseases and their relation to thymectomy. Acta Neurol Scand. 1989. 80: 290-5
266. Tradtrantip L, Yao X, Su T, Smith AJ, Verkman AS. Bystander mechanism for complement-initiated early oligodendrocyte injury in neuromyelitis optica. Acta Neuropathol. 2017. 134: 35-44
267. Tran C, Du Pasquier RA, Cavassini M, Guex-Crosier Y, Meuli R, Ciuffreda D. Neuromyelitis optica following CMV primo-infection. J Intern Med. 2007. 261: 500-3
268. . Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002. 59: 499-505
269. Trebst C, Jarius S, Berthele A, Paul F, Schippling S, Wildemann B. Update on the diagnosis and treatment of neuromyelitis optica: Recommendations of the neuromyelitis optica study group (NEMOS). J Neurol. 2014. 261: 1-6
270. Uribe-San Martin R, Ciampi E, Galilea A, Sandoval P, Miranda H, Mellado P. Neuromyelitis optica spectrum disorders: Profile of a cohort according to the 2015 diagnostic criteria. Rev Neurol. 2017. 65: 193-202
271. Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S. Cytokine and chemokine profiles in neuromyelitis optica: Significance of interleukin-6. Mult Scler. 2010. 16: 1443-52
272. Vahtera T, Haaranen M, Viramo-Koskela AL, Ruutiainen J. Pelvic floor rehabilitation is effective in patients with multiple sclerosis. Clin Rehabil. 1997. 11: 211-9
273. Vaknin-Dembinsky A, Brill L, Kassis I, Petrou P, Ovadia H, Ben-Hur T. T-cell responses to distinct AQP4 peptides in patients with neuromyelitis optica (NMO). Mult Scler Relat Disord. 2016. 6: 28-36
274. Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. Multidisciplinary management of spinal dural arteriovenous fistulas: Clinical presentation and long-term follow-up in 49 patients. Stroke. 2002. 33: 1578-83
275. van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol. 2006. 7: 954-61
276. van Pelt ED, Wong YY, Ketelslegers IA, Hamann D, Hintzen RQ. Neuromyelitis optica spectrum disorders: Comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur J Neurol. 2016. 23: 580-7
277. Vanotti S, Cores EV, Eizaguirre B, Melamud L, Rey R, Villa A. Cognitive performance of neuromyelitis optica patients: Comparison with multiple sclerosis. Arq Neuropsiquiatr. 2013. 71: 357-61
278. Vincent T, Saikali P, Cayrol R, Roth AD, Bar-Or A, Prat A. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. J Immunol. 2008. 181: 5730-7
279. von Glehn F, Jarius S, Penalva de Oliveira AC, Brandão CO, Farias AS, Damasceno A. Aquaporin-4 antibodies are not related to HTLV-1 associated myelopathy. PLoS One. 2012. 7: e39372-
280. Wang H, Dai Y, Qiu W, Zhong X, Wu A, Wang Y. HLA-DPB1 0501 is associated with susceptibility to anti-aquaporin-4 antibodies positive neuromyelitis optica in Southern Han Chinese. J Neuroimmunol. 2011. 233: 181-4
281. Wang H, Wang K, Wang C, Xu F, Qiu W, Hu X. Increased plasma interleukin-32 expression in patients with neuromyelitis optica. J Clin Immunol. 2013. 33: 666-70
282. Wang H, Wang K, Zhong X, Dai Y, Qiu W, Wu A. Notable increased cerebrospinal fluid levels of soluble interleukin-6 receptors in neuromyelitis optica. Neuroimmunomodulation. 2012. 19: 304-8
283. Wang H, Zhong X, Wang K, Qiu W, Li J, Dai Y. Interleukin 17 gene polymorphism is associated with anti-aquaporin 4 antibody-positive neuromyelitis optica in the Southern Han Chinese – A case control study. J Neurol Sci. 2012. 314: 26-8
284. Wang J, Tian Y, Shao Y, Feng H, Qin L, Xu W. Comparison of spontaneous brain activity revealed by regional homogeneity in AQP4-IgG neuromyelitis optica-optic neuritis versus MOG-IgG optic neuritis patients: A resting-state functional MRI study. Neuropsychiatr Dis Treat. 2017. 13: 2669-79
285. Watanabe M, Furusho K, Takahashi T, Tamaoka A. Galactorrhea in a patient with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: A case report and review of the literature. Neurologist. 2015. 20: 101-3
286. Watanabe S, Misu T, Miyazawa I, Nakashima I, Shiga Y, Fujihara K. Low-dose corticosteroids reduce relapses in neuromyelitis optica: A retrospective analysis. Mult Scler. 2007. 13: 968-74
287. Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013. 8: 477-512
288. Wei Q, Yanyu C, Rui L, Caixia L, Youming L, Jianhua H. Human aquaporin 4 gene polymorphisms in Chinese patients with neuromyelitis optica. J Neuroimmunol. 2014. 274: 192-6
289. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015. 85: 177-89
290. Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology. 1999. 53: 1107-14
291. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007. 6: 805-15
292. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006. 66: 1485-9
293. Wingerchuk DM, Weinshenker BG. Neuromyelitis optica: Clinical predictors of a relapsing course and survival. Neurology. 2003. 60: 848-53
294. Wingerchuk DM, Weinshenker BG. The emerging relationship between neuromyelitis optica and systemic rheumatologic autoimmune disease. Mult Scler. 2012. 18: 5-10
295. Wingerchuk DM. Neuromyelitis optica: Current concepts. Front Biosci. 2004. 9: 834-40
296. Wingerchuk DM. Neuromyelitis optica: Effect of gender. J Neurol Sci. 2009. 286: 18-23
297. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013. 496: 513-7
298. Yamamoto T, Mori M, Uzawa A, Uchiyama T, Sakakibara R, Yanagisawa M. Urinary symptoms and neurological disabilities are differentially correlated between multiple sclerosis and neuromyelitis optica. Clin Exp Neuroimmunol. 2016. 7: 52-8
299. Yao X, Verkman AS. Complement regulator CD59 prevents peripheral organ injury in rats made seropositive for neuromyelitis optica immunoglobulin G. Acta Neuropathol Commun. 2017. 5: 57-
300. Yao X, Verkman AS. Marked central nervous system pathology in CD59 knockout rats following passive transfer of neuromyelitis optica immunoglobulin G. Acta Neuropathol Commun. 2017. 5: 15-
301. Yesilot N, Mutlu M, Gungor O, Baykal B, Serdaroglu P, Akman-Demir G. Clinical characteristics and course of spinal cord involvement in Behçet's disease. Eur J Neurol. 2007. 14: 729-37
302. Yoshimura S, Isobe N, Matsushita T, Yonekawa T, Masaki K, Sato S. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry. 2013. 84: 29-34
303. Zaidi SF. Helicobacter pylori associated asian enigma: Does diet deserve distinction?. World J Gastrointest Oncol. 2016. 8: 341-50
304. Zajicek JP, Scolding NJ, Foster O, Rovaris M, Evanson J, Moseley IF. Central nervous system sarcoidosis – Diagnosis and management. QJM. 1999. 92: 103-17
305. Zamvil SS, Spencer CM, Baranzini SE, Cree BA. The gut microbiome in neuromyelitis optica. Neurotherapeutics. 2018. 15: 92-101
306. Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF. A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int. 2015. 6: 171-
307. Zhang H, Verkman AS. Longitudinally extensive NMO spinal cord pathology produced by passive transfer of NMO-IgG in mice lacking complement inhibitor CD59. J Autoimmun. 2014. 53: 67-77
308. Zhao S, Mutch K, Elsone L, Miller J, Jacob A. An unusual case of ‘itchy paralysis’: Neuromyelitis optica presenting with severe neuropathic itch. Pract Neurol. 2015. 15: 149-51
309. Zhao S, Mutch K, Elsone L, Nurmikko T, Jacob A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. 2014. 20: 1658-61
310. Zhong YH, Zhong ZG, Zhou Z, Ma ZY, Qiu MY, Peng FH. Comparisons of presentations and outcomes of neuromyelitis optica patients with and without Sjögren's syndrome. Neurol Sci. 2017. 38: 271-7