- Department of Neurosurgery, Zagazig University, Zagazig, Egypt.
Correspondence Address:
Mohamed Salah, Department of Neurosurgery, Zagazig University, Zagazig, Egypt.
DOI:10.25259/SNI_1131_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohamed Salah1, Ahmed Shalaby1. Computed tomography-guided stereotactic surgery in the management of brain lesions: A single-center experience. 26-May-2023;14:184
How to cite this URL: Mohamed Salah1, Ahmed Shalaby1. Computed tomography-guided stereotactic surgery in the management of brain lesions: A single-center experience. 26-May-2023;14:184. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12335
Abstract
Background: The present study presents our experience with computed tomography (CT)-guided stereotactic surgery in managing deep-seated brain lesions and provides a background in the expanding fields of morphological stereotactic neurosurgery.
Methods: We conducted this retrospective cohort study on 80 patients managed at the Department of Neurosurgery, Zagazig University Hospitals, Zagazig, Egypt, between January 2019 to January 2021. We targeted patients with morphological stereotactic surgeries performed as the primary management modality of their treatment.
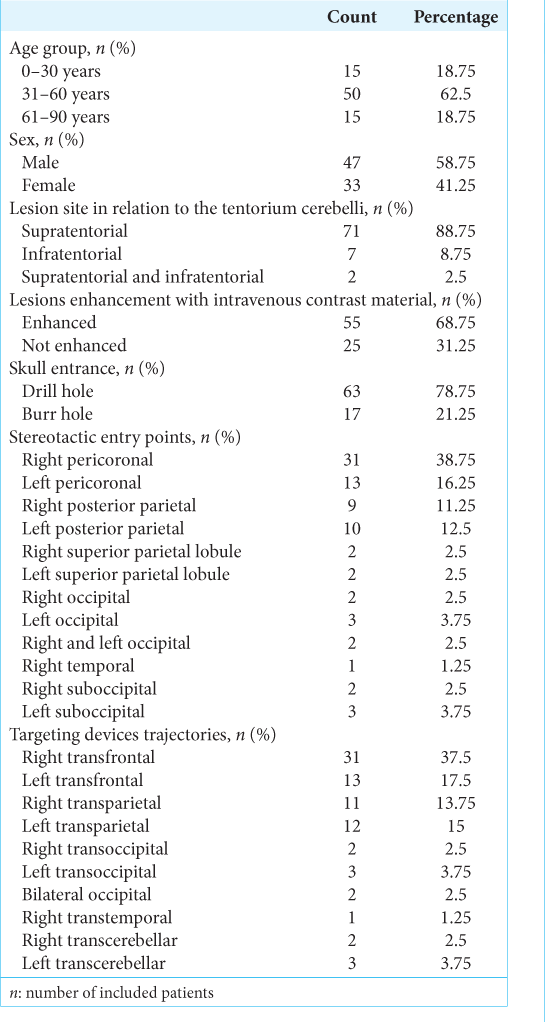
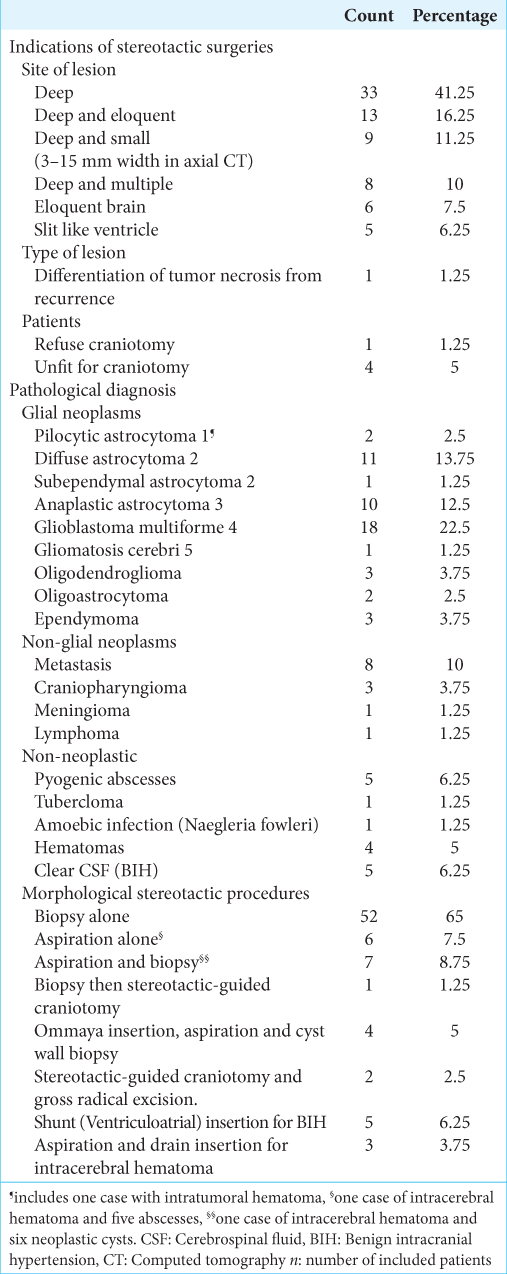
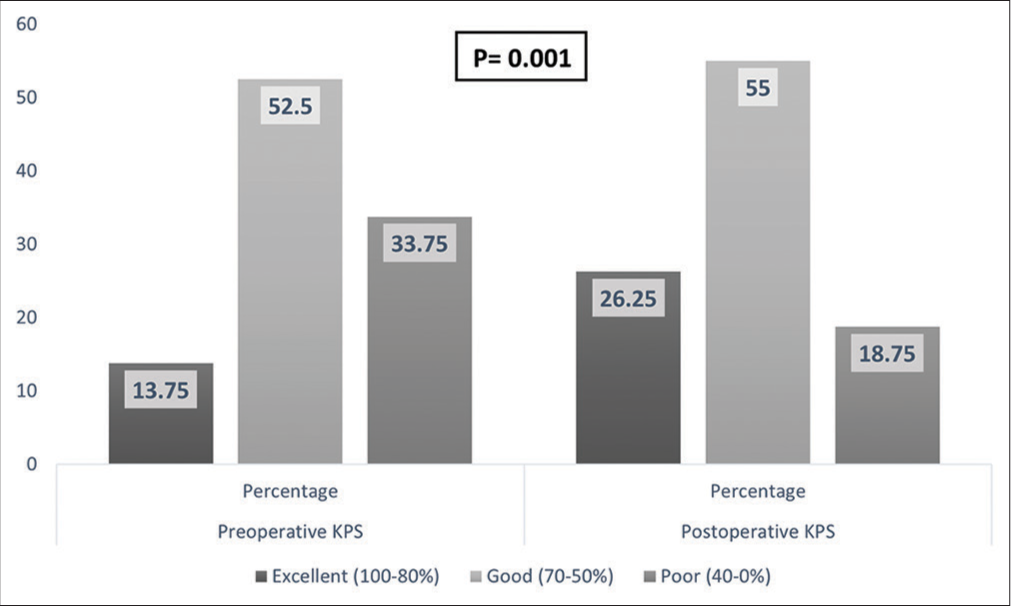
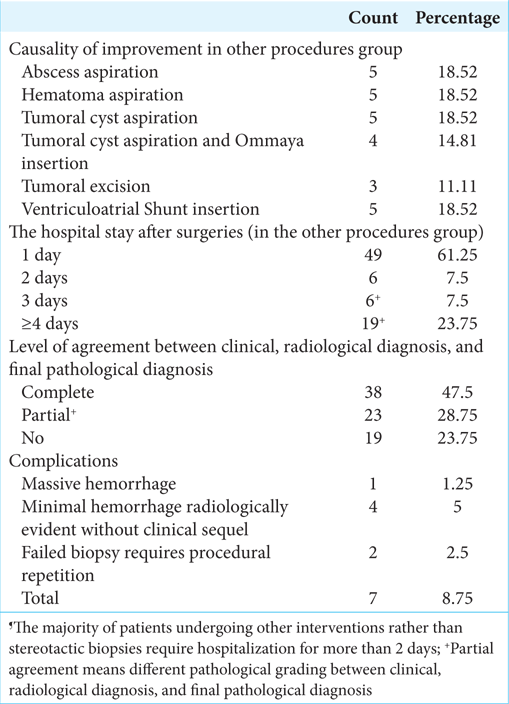
Results: A total of 80 patients, with a mean age of 44.3 years, were included in the study. The stereotactic targets were supratentorial in 71 patients (88.75%), infratentorial in seven patients (8.75%), and both supraand infratentorial in two patients (2.5%). The lesions showed enhancements with IV contrast in 55 patients (68.75%). Stereotactic procedures were performed under local anesthesia in 64 patients and general anesthesia in 16 patients. Of the 80 stereotactic procedures, 52 were biopsies (65%). We observed a significant improvement in the postoperative Karnofsky performance score compared to the postoperative score (63.4 ± 19.8 vs. 56.7 ± 15.4, P = 0.001). The level of agreement between clinical, radiological, and final pathological diagnosis was assessed; it was complete in 47.5% of the patients. The postprocedural CT scan demonstrated intracranial hemorrhage in five patients (6.25%); four (5%) were silent with no neurological complications.
Conclusion: This study provided evidence that the stereotactic procedure is easy to perform, accurate in targeting the lesion, and spares patients from undergoing major surgical procedures. Stereotactic applications of spontaneous intracerebral hemorrhage, deep-seated abscesses, encysted tumors, or medically refractory benign intracranial hypertension can improve the outcome even in medically high-risk patients.
Keywords: Deep-seated brain lesions, Neurosurgery, Histopathology, Stereotactic surgery
INTRODUCTION
The importance of stereotactic localization tools in modern neurosurgery is equal to that of other valuable standard devices, such as the ultrasonic aspirator, the laser, and the operating microscope in microsurgery.[
For patients with minimal brain metastases and good performance status, stereotactic radiation is the standard treatment.[
The majority of brain lesions can be biopsied and classified according to their pathological nature using a standard stereotactic brain biopsy technique with a high diagnostic yield.[
During the postradiosurgery follow-up, magnetic resonance perfusion imaging, particularly dynamic contrast-enhanced (DCE), aids in the differential detection of tumor recurrence and RN. Some instances of RN cannot be reliably distinguished from tumors using CT or MRI (especially astrocytoma; RN occasionally resembles glioblastoma). To identify RN from tumor recurrence, MRI perfusion-based techniques such as DCE dynamic susceptibility-weighted imaging and magnetic resonance spectroscopy (MRS) have been utilized.[
Nonetheless, susceptibility artifacts and the small lesion size were most likely responsible for the equivocal perfusion-weighted imagingMRI and MRS results. The preferred approach for monitoring brain metastases following SRS therapy is contrast-enhanced structural MRI. Nevertheless, in many cases, utilizing contrast-enhanced MRI to differentiate local recurrent brain metastasis from radiation-induced alterations following SRS is problematic.[
Although stereotactic surgery is minimally invasive, the procedure has definite risks that must be contemplated for each patient against the benefits. The incidence of morbidity due to stereotactic biopsy ranges from 1% to 6.5%, and mortality rates range from 0% to 1.7% in referenced large studies.[
MATERIALS AND METHODS
We conducted this retrospective cohort study on 80 patients managed at the Department of Neurosurgery, Zagazig University Hospitals, Zagazig, Egypt, between January 2019 and January 2021. We targeted patients with morphological stereotactic surgeries performed as the main management modality of their treatment. Our study protocol was approved by the Hospital Research and Ethics Committee at the Faculty of Medicine, Zagazig University (#10162). Routinely, after understanding the procedure’s purpose, benefits, and potential adverse events, each patient voluntarily provided written informed permission.
Eligibility criteria
Inclusion criteria
The inclusion criteria that were employed to ensure that the patients are suitable for such a technique of surgery included in the study:
Lesions in functionally critical areas, such as motor, sensory cortex, or basal ganglia Invasive neoplastic lesions without mass effect or significant neurological signs Poorly defined lesions on CT or MRI Small lesions and deep-seated lesions, as in the brain stem or midline region Multiple lesions, where a distinction between metastasis and inflammatory lesions is required Patients with poor medical conditions or advanced age who cannot tolerate prolonged craniotomy Lesions in which differentiation between tumors recurrence and radio necrosis is required Deep-seated subcortical brain infections Medically refractory benign intracranial hypertension (BIH) patients with slit-like lateral ventricles for insertion of ventricular shunt Patients with cystic deep-seated neoplastic or nonneoplastic lesions, for aspiration and/or insertion of Ommaya reservoir Stereotactic-guided craniotomy for different brain lesions Patients with intra-axial deep-seated spontaneous hematomas without brain stem extension with clinical onset 48 h and more than 25 mL in volume for aspiration and/or insertion of temporary drainage catheter for intracavitary injection of thrombolytic medication to evacuate residual hematoma if presented Radiosensitive lesions such as germ cell tumors and lymphomas.
Exclusion criteria
We excluded patients with any of the following conditions:
Patients with suspected vascular lesions such as vascular malformations or cerebral aneurysms Patients with extensive brain lesions with a significant mass effect require open surgery and decompression Patients with lesions in close relation to large brain cisterns, especially suprasellar cistern, for a high possibility of aneurysms Patients with uncorrectable coagulation disorders Patients with a Glasgow coma score <8 Patients with signs of tentorial herniation Patients who were unable to provide informed consent Infants or children below 2 years of age Patients with an intracranial device that interferes with trajectory pathway.
Study process
All patients underwent complete history taking followed by a general examination. The Karnofsky performance score (KPS) was used to assess the functional outcome [Appendix S1]. To document the accuracy and precision of intervention, postoperative CT is usually performed 5 h after the procedure. This enables a comparison of the intended target coordinates with the actual target coordinates of the surgical intervention and the detection of suspected complications.
Technique
Following preoperative assessment and preparation, we applied the base ring of Leksell’s frame “G” generation (Elekta Instruments, Stockholm, Sweden) to the patient’s head after infiltration of pins site with local anesthetic medication (xylocaine 2%, dose 5 mg/kg and adrenaline 1:100,000). A low set frame was preferable to avoid aligning the stereotactic frame pins with the target plane to avoid the metallic artifact that would obscure the image. The patients were transported to the scanner room, with an anesthesiologist and neurosurgeon in attendance. Scan orientation was parallel to the basal ring, axial images, 2 mm apart, and the scan gantry was 0. We usually administer intravenous contrast (iohexol, omnipaque 300 mg/mL at dose 100–150 mL for adults and 1–2 mL/kg for children) except for those with iodine allergy or renal dysfunction. For unenhanced lesions, we administrate an additional 50–70 mL of omnipaque 300 and repeat the scan immediately and in a delayed fashion. The contrast would delineate the enhanced lesion and show the nearby blood vessels to be avoided during trajectory planning, even if the lesion is not enhancing. Slices disturbed with metallic artifacts were neglected; we chose a clear-cut with all visible fiducials. We preferred the local anesthesia with a neuroanesthesiologist standing by. General anesthesia was indicated for patients requiring stereotactic-guided craniotomy and insertion of brain shunts and for patients with severe continuous movements, severe pain, scoliosis, vertebral pain, heavy coughing, and anxiety, or those who are uncooperative and children. Biopsies were pushed out from Sedan type needle by a saline jet and laid on a test tube with formalin 10%. We usually take 2–7 specimens except for the brain stem lesions; in such cases, we only take one bite under considerable negative pressure. We sent the specimen to an experienced neuropathologist and requested an examination of all biopsy fragments.
Statistical analysis
All analyses were performed using the Statistical Package of the Social Sciences (SPSS) version 22 (SPSS Inc., Chicago, Illinois, USA). We presented the qualitative data in frequencies and percentages. Continuous data were presented as mean ± standard deviation or median and range for parametric and non-parametric data. The paired t-test was used to assess the statistically significant difference between the two population means of dependent (paired) samples. The significant difference was considered when P < 0.05.
RESULTS
Demographics of the include patients
A total of 80 patients (47 males and 33 females), with a mean age of 44.3 years (range 5–87 years), were included in the present study. Most patients aged between 31 and 60 years (62.5%). All patients underwent preoperative brain CT scans and MRI for target localization. The stereotactic targets were supratentorial in 71 patients (88.75%), of which five cases were BIH, infratentorial in seven patients (8.75%), and both supra- and infratentorial in two patients (2.5%) [Appendix S2]. The lesions showed enhancements with IV contrast in 55 patients (68.75%). Entry techniques included twist-drill craniostomy as well as burr-hole craniostomy. The drill hole technique was performed in 63 patients (78.75%), while the burr-hole technique was utilized for stereotactically guided craniotomies, hematoma evacuation, and shunt or Ommaya insertions. Trajectories chosen for the stereotactic procedures depended on the site of the lesions and the nature of the procedure, as shown in
Further assessment dividing the patients into biopsy alone group and other procedures group showed no significant difference between the two groups in terms of preoperative KPS (P = 0.9) and showed a significant difference between the two groups in postoperative KPS (P = 0.001). When the preoperative and postoperative KPS were compared, the other procedures group showed significant improvement (P = 0.001), but not the biopsy alone group (P = 0.73), [Appendix S3].
In the other procedures group, the improvement causes were abscess aspiration, hematoma aspiration, tumoral cyst aspiration, tumoral cyst aspiration with Ommaya Reservoir insertion, tumoral excision, and ventriculoatrial shunt insertion. Most patients (n = 49, 61.25%) stayed for 1 day, while 23.75% stayed for ≥4 days. The level of agreement between clinical, radiological, and final pathological diagnosis was assessed; it was complete in 47.5% of the patients. The postprocedural CT scan demonstrated intracranial hemorrhage in five patients (6.25%); four (5%) were silent with no neurological complications. Postoperative data are shown in
DISCUSSION
In our study, preoperative brain CT and MRI were performed for target localization. Several methods have been offered to increase the accuracy, and diagnostic yield of the CT-guided stereotactic brain biopsy, such as targeting multiple regions of the lesion as most brain tumors harbor different pathological grading like astrocytoma, delaying the localization scan after the administration of contrast medium to improve resolution and target selection, proper tissue handling, utilizing modern histopathological techniques, and double-check of stereotactic coordinates calculation and registration to avoid technical errors.[
Stereotactic procedures were performed under local and general anesthesia in 64 and 16 patients, respectively. The main indications for general anesthesia were young age, uncooperative patients, and the need for shunt insertions, lesions resection, or hematoma evacuation. About 65% of the stereotactic procedures were biopsied. Stereotactic biopsy is a minimally invasive diagnostic procedure with minimal risk for patients.[
A histopathological analysis is important when the treatment of brain lesions is planned. The stereotactic brain biopsy is indicated for the diagnosis of inaccessible deep-seated lesions, lesions in the eloquent areas of the brain, diffuse infiltrative brain lesions with minimal to moderate mass effect, multiple and cystic lesions, and for patients with poor medical conditions for a craniotomy. In these patients, stereotactic biopsy provides a small tissue sample from a target point predetermined by radiological methods with low morbidity and mortality rates.[
In our study, astrocytoma was the commonest pathology, followed by brain metastasis. We identified three levels of agreement between pre-biopsy clinical and radiological diagnosis and the final pathological diagnosis. Complete agreement was noticed in 47.5% of the included patients, partial agreement in 28.75%, and no agreement at all in 23.75%. The important role of stereotactic biopsy is confirming tissue diagnosis for patients with multiple brain lesions in a negative systemic metastatic survey setting. In our series, four out of eight patients with multiple brain lesions were primary brain tumors. A retrospective review by Arbit and Galicich found that 19% of cases that underwent stereotactic biopsy had a different diagnosis than radiographic.[
Postprocedural increasing of cerebral edema was documented as the commonest finding in follow-up CT brain.[
In our study, we successfully evacuated five intracerebral hematomas; four were thalamic without intraventricular extension, and one was frontoparietal. All of them have improved. We used the Backlund hematoma evacuator kit with drill screws in all patients. Sufficient reduction of hematoma volume without fibrinolysis is achieved in two cases. Furthermore, residual hematomas were liquefied by streptokinase infusion (6000 IU) and drained through a catheter, usually placed in a hematoma center for three patients. Our patients have no rebleeding as we keep systolic blood pressure not more than 150 mmHg in operative and postoperative times. We never aspirate the residual hematoma after fibrinolytic agent infusion to avoid negative pressure inside the hematoma cavity. We only let the hematoma drain spontaneously against 0 cm of pressure. By that time, a maximal mass effect from surrounding edema would have been anticipated, and there was no clinical deterioration from edema or mass associated with residual hematoma.
In our study, five patients with six supratentorial deep-seated abscesses underwent stereotactically guided aspiration. Antibiotics were started as soon as the procedure was finished. Antibiotic combination therapy was adjusted according to the results of cultures and sensitivity tests. Follow-up was performed with clinical evaluation and repeated CT scans for three to 6 months. All patients had normal courses, and no recurrence was observed. All patients returned to their previous activities within a median of 3 months after the operation.
Kondziolka et al. reported a 93% success rate in the stereotactic treatment of brain abscesses.[
The present study showed ten neoplastic cystic lesions (two peritumoral cysts with mural nodules and eight intratumoral cystic lesions). All patients had one CT-guided stereotactic cyst aspiration. Four patients (three craniopharyngiomas and one recurrent anaplastic astrocytoma cyst) required a catheter-reservoir system. Histopathological analysis revealed three craniopharyngiomas, two pilocytic astrocytomas, three glioblastoma multiforme, one anaplastic astrocytoma, and one metastatic adenocarcinoma. Symptomatic improvement was achieved in nine patients. A silent intracystic hemorrhage occurred in one patient after a cyst wall biopsy. All patients undergoing Ommaya reservoir insertions were symptomatically improved with significant tumor size control. No procedure-related mortality was encountered. Niranjan et al. reported that 38 patients with glial and metastatic brain cysts were managed with single stereotactic aspiration. Twelve patients of them required an intracavity catheter and Ommaya reservoir insertion. All patients were symptomatically improved.[
CONCLUSION
This study provided evidence that the stereotactic procedure is easy to perform, accurate in targeting the lesion, and spares patients from undergoing major surgical procedures. The specimen taken for biopsy was adequate for diagnosis.
Stereotactic target localization is more than 97.5% if the meticulous methodology is applied. Stereotactic applications of spontaneous ICH, deep-seated abscesses, encysted tumors, or medically refractory BIH are minimally invasive, highly effective, and accurate, which can improve the outcome even in medically high-risk patients. Overall, complications arising from stereotactic surgeries are infrequent, with minimal associated morbidity and mortality compared to other cranial surgical procedures. Given the current state of the art, frame-based surgeries are still an important technique, even in the frameless era.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aker FV, Hakan T, Karadereler S, Erkan M. Accuracy and diagnostic yield of stereotactic biopsy in the diagnosis of brain masses: Comparison of results of biopsy and resected surgical specimens. Neuropathology. 2005. 25: 207-13
2. Amundson EW, McGirt MJ, Olivi A. A contralateral, transfrontal, extraventricular approach to stereotactic brainstem biopsy procedures. J Neurosurg. 2005. 102: 565-70
3. Ansari M, Jha S. Tuberculous brain abscess in an immunocompetent adolescent. J Nat Sci Biol Med. 2014. 5: 170-2
4. Arbit E, Galicich JH. Importance of image-guided stereotactic biopsy to confirm diagnosis in an oncological setting. Ann Surg Oncol. 1994. 1: 368-72
5. Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Am J Neuroradiol. 2009. 30: 367-72
6. Bernays RL, Kollias SS, Khan N, Brandner S, Meier S, Yonekawa Y. Histological yield, complications, and technological considerations in 114 consecutive frameless stereotactic biopsy procedures aided by open intraoperative magnetic resonance imaging. J Neurosurg. 2002. 97: 354-62
7. Bisdas S, Naegele T, Ritz R, Dimostheni A, Pfannenberg C, Reimold M. Distinguishing recurrent high-grade gliomas from radiation injury. Acad Radiol. 2011. 18: 575-83
8. Boulton M, Bernstein M. Outpatient brain tumor surgery: Innovation in surgical neurooncology. J Neurosurg. 2008. 108: 649-54
9. Brainard JA, Prayson RA, Barnett GH. Frozen section evaluation of stereotactic brain biopsies: Diagnostic yield at the stereotactic target position in 188 cases. Arch Pathol Lab Med. 1997. 121: 481-4
10. Broggi G, Franzini A, Peluchetti D, Servello D. Treatment of deep brain abscesses by stereotactic implantation of an intracavitary device for evacuation and local application of antibiotics. Acta Neurochir (Wien). 1985. 76: 94-8
11. Çalişaneller T, Özdemir Ö, Özger Ö, Özen Ö, Kiyici H, Caner H. The accuracy and diagnostic yield of computerized tomography guided stereotactic biopsy in brain lesions. Turk Neurosurg. 2008. 18: 17-22
12. Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009. 10: 1037-44
13. Chao ST, Ahluwalia MS, Barnett GH, Stevens GH, Murphy ES, Stockham AL. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol. 2013. 87: 449-57
14. Chen CC, Hsu PW, Wu TW, Lee ST, Chang CN, Wei KC. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009. 111: 835-9
15. Dade Lunsford L, Martinez AJ. Stereotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984. 22: 222-30
16. Dammers R, Schouten JW, Haitsma IK, Vincent AJ, Kros JM, Dirven CM. Towards improving the safety and diagnostic yield of stereotactic biopsy in a single centre. Acta Neurochir (Wien). 2010. 152: 1915-21
17. Dooms GC, Hecht S, Brant-Zawadski M. Brain radiation lesions: MR imaging. Radiology. 1986. 158: 149-55
18. Ferreira MP, Ferreira NP, Filho AD, Filho GD, Franciscatto AC. Stereotactic computed tomography-guided brain biopsy: Diagnostic yield based on a series of 170 patients. Surg Neurol. 2006. 65: S27-32
19. Fritsch MJ, Leber MJ, Gossett L, Lulu BA, Hamilton AJ. Stereotactic biopsy of intracranial brain lesions. High diagnostic yield without increased complications: 65 consecutive biopsies with early postoperative CT scans. Stereotact Funct Neurosurg. 1998. 71: 36-42
20. Gildenberg PL, Kelly PJ, Goodrich JT, Kondziolka D, Tasker RR. The birth of stereotactic surgery: A personal retrospective. Neurosurgery. 2004. 54: 199-208
21. Grossman R, Sadetzki S, Spiegelmann R, Ram Z. Haemorrhagic complications and the incidence of asymptomatic bleeding associated with stereotactic brain biopsies. Acta Neurochir (Wien). 2005. 147: 627-31
22. Grossman R, Schmidek HH, Quiñones-Hinojosa A, editors. Management of suppurative intracranial infections. Schmidek and Sweet Operative Neurosurgical Techniques: Indications. Methods, and Results. Netherlands: Elsevier Inc; 2012. 2: 1631-41
23. Hernes TA, Ommedal S, Lie T, Lindseth F, Langø T, Unsgaard G. Stereoscopic navigation-controlled display of preoperative MRI and intraoperative 3D ultrasound in planning and guidance of neurosurgery: New technology for minimally invasive image-guided surgery approaches. Minim Invasive Neurosurg. 2003. 46: 129-37
24. Hsieh PC, Pan HC, Chung WY, Lee LS. Computerized tomography-guided stereotactic aspiration of brain abscesses: Experience with 28 cases. Zhonghua Yi Xue Za Zhi (Taipei). 1999. 62: 341-9
25. Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK. Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol. 2006. 23: 71-5
26. Jain D, Sharma MC, Sarkar C, Gupta D, Singh M, Mahapatra AK. Comparative analysis of diagnostic accuracy of different brain biopsy procedures. Neurol India. 2006. 54: 394-8
27. Kalinowska-Nowak A, Garlicki A, Bociaga-Jasik M. Brain abscess--modern diagnostics and therapeutic treatment. Przegl Epidemiol. 2009. 63: 89-95
28. Kepes JJ. Pitfalls and problems in the histopathologic evaluation of stereotactic needle biopsy specimens. Neurosurg Clin N Am. 1994. 5: 19-33
29. Kondziolka D, Duma CM, Lunsford LD. Factors that enhance the likelihood of successful stereotactic treatment of brain abscesses. Acta Neurochir (Wien). 1994. 127: 85-90
30. Kongkham PN, Knifed E, Tomber MS, Bernstein M. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008. 35: 79-84
31. Kutlay M, Çolak A, Yldz Ş, Demircan N, Akn ON. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery. 2008. 62: 540-6
32. Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010. 96: 45-68
33. Mamelak AN, Mampalam TJ, Obana WG, Rosenblum ML. Improved management of multiple brain abscesses: A combined surgical and medical approach. Neurosurgery. 1995. 36: 76-86
34. Manoj N, Arivazhagan A, Bhat D, Arvinda H, Mahadevan A, Santosh V. Stereotactic biopsy of brainstem lesions: Techniques, efficacy, safety, and disease variation between adults and children: A single institutional series and review. J Neurosci Rural Pract. 2014. 5: 32-9
35. McGirt MJ, Villavicencio AT, Bulsara KR, Friedman AH. MRI-guided stereotactic biopsy in the diagnosis of glioma: Comparison of biopsy and surgical resection specimen. Surg Neurol. 2003. 59: 279-83
36. McGirt MJ, Woodworth GF, Coon AL, Frazier JM, Amundson E, Garonzik I. Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J Neurosurg. 2005. 102: 897-901
37. Mohanty A. Biopsy of brain stem gliomas: Changing trends?. J Neurosci Rural Pract. 2014. 5: 116-7
38. Niranjan A, Witham T, Kondziolka D, Lunsford LD. The role of stereotactic cyst aspiration for glial and metastatic brain tumors. Can J Neurol Sci. 2000. 27: 229-35
39. Nishihara M, Kohmura E, Takeda N, Harada T, Kidoguchi K, Tatsumi S. Diagnostic yield and morbidity by neuronavigation-guided frameless stereotactic biopsy using magnetic resonance imaging and by frame-based computed tomography-guided stereotactic biopsy. Surg Neurol Int. 2014. 5: S421-6
40. Nishihara M, Sasayama T, Kudo H, Kohmura E. Morbidity of stereotactic biopsy for intracranial lesions. Kobe J Med Sci. 2010. 56: E148-53
41. Owen CM, Linskey ME. Frame-based stereotaxy in a frameless era: Current capabilities, relative role, and the positive-and negative predictive values of blood through the needle. J Neurooncol. 2009. 93: 139-49
42. Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014. 15: 11832-46
43. Rogers LR, Barnett G. Percutaneous aspiration of brain tumor cysts via the ommaya reservoir system. Neurology. 1991. 41: 279-82
44. Schlemmer HP, Bachert P, Henze M, Buslei R, Herfarth K, Debus J. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology. 2002. 44: 216-22
45. Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016. 7: 12318-30
46. Takahashi H, Sugai T, Uzuka T, Kano M, Honma J, Grinev I. Complications and diagnostic yield of stereotactic biopsy for the patients with malignant brain tumors. Neurol Surg. 2004. 32: 135-40
47. Thomas DG, editors. Stereotactic biopsies for tumors: Indications, limits, diagnosis with histopathology and other laboratory techniques. Practical Handbook of Neurosurgery. Berlin: Springer Verlag; 2009. p. 529-47
48. Woodworth GF, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Frameless image-guided stereotactic brain biopsy procedure: Diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006. 104: 233-7
49. Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: Comparison of biopsy and open resection specimen. Neurol Res. 2005. 27: 358-62
50. Zrinzo L. Pitfalls in precision stereotactic surgery. Surg Neurol Int. 2012. 3: S53-61