- Department of Neurosurgery, Hospital Kuala Lumpur, Kuala Lumpur, Wilayah Persekutuan Kuala Lumpur,
- Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia.
Correspondence Address:
Chun Lin Lee
Department of Neurosurgery, Hospital Kuala Lumpur, Kuala Lumpur, Wilayah Persekutuan Kuala Lumpur,
DOI:10.25259/SNI_64_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Chun Lin Lee, Regunath Kandasamy, Mohammed Azman Bin Mohammad Raffiq. Computed tomography perfusion in detecting malignant middle cerebral artery infarct. 09-Aug-2019;10:159
How to cite this URL: Chun Lin Lee, Regunath Kandasamy, Mohammed Azman Bin Mohammad Raffiq. Computed tomography perfusion in detecting malignant middle cerebral artery infarct. 09-Aug-2019;10:159. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9571
Abstract
Background: Computed tomography perfusion (CTP) is an emerging modality which produces maps of time-to- peak (TTP), cerebral blood flow (CBF), and cerebral blood volume (CBV), with a computerized automated map of the infarct and penumbra. This modality provides a better evaluation of the extent of infarction, making it a potential method for assessing patients suffering from large middle cerebral artery (MCA) infarctions.
Methods: A prospective cohort study of all patients in Hospital Kuala Lumpur, Malaysia, who presented with the clinical diagnosis of a large MCA infarction within 48 h of onset were subjected to CT brain, and CTP scans on admission and were followed up to determine the development of malignant infarction requiring surgical decompression.
Results: CTP parameters were generally lower in patients with malignant brain infarct (MBI) group compared to the nonMBI group. The largest mean difference between the group was noted in the TTP values (P = 0.005). CTP parameters had a comparable positive predictive value (83%–90%) and high net present value (88–93). CBF with cutoff value of >32.85 of the hemisphere could accurately predict malignant infarctions in 81.4% of cases. The National Institutes of Health Stroke Scale score of more than 13.5 was also found to be able to accurately determine malignant infarct (97.6%). Functional outcome of patients based on Glasgow outcome scale was similar on discharge, however, showed improvement at 6 months during reviewed base on modified Rankin scale (P
Conclusion: CTP parameters should be included in the initial evaluation of patients to predict malignant brain infarction and facilitate surgical treatment of large MCA infarctions.
Key messages: CT perfusion parameters have an important role in predicting malignant brain infarction and should be included in the initial evaluation of patients to facilitate the early identification and surgical treatment of large middle cerebral artery infarctions, to improve patient’s prognosis.
Keywords: Malignant brain infarction, Middle cerebral artery, Perfusion computed tomography, Stroke
INTRODUCTION
Acute ischemic stroke is a leading cause of morbidity and long-term rehabilitation in adults. Acute ischemic stroke initiates a pathophysiological cascade that leads to the formation of brain edema. Malignant middle cerebral artery (MCA) infarction is a clinical entity affecting up to 10% of all patients diagnosed with ischemic stroke. It is defined as an infarction involving an area encompassing at least two-thirds of that supplied by the MCA.[
Decompressive hemicraniectomy, however, remains the mainstay of treatment in cases of space occupying edema with rapid clinical deterioration secondary to raise ICP.
Astrup et al. in 1981 first coined the term ischemic penumbra, defined as tissue within the threshold of functional impairment and morphological integrity which has the capacity to recover if blood flow is restored timely, surrounding the area of ischemic core which remains refractory to reperfusion. Identification and differentiation of these regions; core versus penumbra is critical in the evolution and treatment of patients with acute ischemic stroke.
The outcome in patients with stroke; in particularly, malignant MCA infarction is dependent on two principal factors; the severity of stroke on presentation and the speed of intervention. Thus, early and accurate diagnosis plays a crucial in determining outcome. Radiological investigation remains the primary diagnostic modality used in the triage of stroke patients on initial presentation; with computed tomography (CT) scan and magnetic resonance imaging (MRI) being the principle workhorses; but neither without its limitations. CT scan, although being fast in confirming diagnosis lacks sensitivity and the opposite applies to MRI. These limitations lead to a delay in accurate diagnosis and unfavorable outcome. To overcome this limitation, recent publications favours the use of CT Perfusion as an additional triage tool; bridging the gap between fast data acquisition of a CT scan and sensitivity of detection of MRI.
This study aims to explore the qualitative (and quantitative) benefits of CTP and potential predictive ability in diagnosing malignant MCA infarction.
Background
Outcome following treatment of strokes; particularly of malignant MCA infarction is dependent on various factors, including age of the patient; progression of symptoms, infarct volume, development of space occupying edema, presence of mass effect on CT scan, and interval between ictus and intervention. Central to the challenge of improving the outcome of malignant MCA infarction is an early and accurate diagnosis. Identifying patients at risk for developing space occupying edema are vital; particularly in the aspect of clinical decision making of treatment. The extent of ischemia cannot be assessed by neurological examination alone. Multiple trials, meta-analysis and opinions have been published describing early predictors of malignant MCA infarction. These include clinical, biochemical and radiological parameters, all aimed to improve the diagnostic accuracy of this life-threatening condition. Clinical parameters used are commonly the Glasgow coma scale (GCS) scoring system; and the National Institutes of Health Stroke Scale (NIHSS). Both these scoring scales have a strong correlation in predicting of outcome in patients with malignant MCA infarction. The GCS score is in most cases of triage, the primary tool of assessment in patients with neurological dysfunction. Weir et al noted that GCS score had a strong predictive value for early mortality and functional outcome in patients with malignant infarction.[
Wartenberg et al. have identified several predictors development of brain edema in MCA infarction; namely history of hypertension (HTN), history of congestive heart failure, elevated white cell count, involvement of MCA infarction territory >50%, and involvement of other vascular territories.[
Collateral circulation has been associated with recanalization, infarct volume, and clinical outcome in patients undergoing acute reperfusion therapies.
CT based evaluation of collaterals offers a promising alternative for assessing the ischemic injury. The pial collateral circulation limits the core infarct size by supporting penumbral tissue during acute ischemia. Multiple studies have evaluated collaterals using CT angiography and have demonstrated improved tissue and clinical outcomes in patients with more robust CTA collaterals. In patients with acute ischemic stroke of terminal ICA or proximal MCA occlusions, the degree of collateral circulation on admission CTA correlates with the admission diffusion-weighted imaging (DWI) lesion volume. However, due to their poor specificity, these grading systems are not particularly helpful for treatment decisions in individual patients.
A noncontrast CT scan has remained the mainstay in the evaluation of acute ischemic stroke chiefly as it is fast, cheap, effective and widespread availability, and good interobserver variability.[
Although very sensitive in detecting hemorrhage in stroke, CT scan lacks the sensitivity in early detection of ischemic stroke; appearance and progression of infarct and edema are typically seen after an interval of 3–4 h postictus; resulting in a significant delay in treatment and increasing risk of morbidity and mortality.
MRI brain is superior in terms of early detection of malignant MCA infarction with cerebral edema and remains the gold standard in determining penumbra. Use of parametric MRI with DWI and perfusion-weighted imaging has defined the standards of diagnosing space occupying edema in malignant MCA infarction. In the study by Oppenheim et al. used DWIs performed within 14 h after stroke onset for a small series of patients, 10 patients with a malignant course and 18 patients with a nonmalignant course, a DWI lesion volume more than 145 ml was proposed as a predictor of a malignant hemispheric infarction with a 94% specificity, 100% sensitivity, and 91% positive predictive value (PPV).[
Evoking the concept of time is neurons, a heightened interest in identifying predictive markers of malignant infarction. Various clinical and biochemical markers have been proven to be sensitive markers, but they lack the required specificity. Radiological parameters remain the mainstay of diagnosis in the clinical setting of acute ischemic stroke. The ideal imaging modality in the triage of patients should be rapid; accurate and capable of identifying and differentiating areas of tissue infarction from areas of ischemic penumbra. Multi- parametric MRI remains the gold standard in identifying areas of infarct versus areas of risk, but limited due to technical and logistical acumen; while CT scan despite being rapid is inaccurate and incapable of detecting hyperacute stroke and areas of the penumbra.
Cerebral CTP measures the cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT). CBF is a quantitative measurement of blood that perfuses 100 g of the brain in per unit of time (ml/100 g/min); CBV is the amount of blood in a given volume of brain (ml/100 ml); while MTT is the time taken for the blood to pass through the brain tissue. The total volume of flowing blood in a given brain volume is CBV, the total volume of blood passing through a given volume of brain per unit time is defined as CBF; and finally, the average time taken for blood passing through a given brain region is MTT.[
CTP fulfills the required model of imaging standard; in such that it offers rapid data acquisition and image analysis; and allows identification of penumbra region. The basic principle of CTP is tracing of a bolus of iodinated contrast material through the cerebral circulation through sequential spiral CT scanning in Cine Mode. This technique allows measurement of various parameters; namely CBF (normal 50 ml/100 g brain tissue/min); CBV (ml/100 g brain tissue) time to peak (TTP) (delay between intravenous administration and peak concentration at brain parenchyma); and MTT (mean time for blood to travel through brain parenchyma). These parameters reflect hemodynamic parameters with brain circulation and detect any difference or changes that occur.
In normal brain; all parameters are symmetrical; reflecting normal CBF and physiology. In acute infarction, areas of core infarction demonstrate a decrease in CBF and CBV, with increase MTT and TPP. Areas of decrease CBV (<30%) accurately reflect areas of core infarct when compared to DWI MRI.[
Thus, CTP in a way is a form of physiologic imaging, reflecting active cerebrovascular physiology derived from multiple parameters; rather than a single parameter of hypodensity of signal intensity changes. Conventionally, CTP has been devised as a tool of qualitative assessment; done through simple visual study. The recent development of software has expanded its utility into quantitative measurement; and development of CTP threshold values depicting areas of infarct core and penumbra. These thresholds, however, remain to be validated.
In our center; CT scan remains the mainstay of triage in patients with ischemic stroke. All patients with suspected acute stroke undergo CT scan on admission; with the also measuring cerebral perfusion during the initial CT. Therefore, our study focused on CTP to identify the predictive factors of malignant brain edema in MCA infarction patients.
This study was done to determine the predictive value of CTP in cases of malignant MCA infarction. Using the permeability maps generated by CTP; we aim to identify imaging parameters most valuable in detecting tissue at risk and to identify the perfusion threshold for predicting the development of infarction.
Scientific rationale
Problem statement
Brain imaging with CTP has promising potential in providing valuable information regarding the importance of identifying malignant MCA infarct and current problems of its identification, which is vital to treatment decisions.
Importance and validity of research
The volume of patient admission due to acute stroke, especially MCA infarct has seen a steady increase over the years. To date, no quantitative studies have been performed to evaluate permeability maps by means of CTP and to establish its correlation with progression of clinical course. In Hospital Kuala Lumpur; all patients presenting with stroke undergo a CT scan as a primary diagnostic tool on admission, principally to identify the neurological pathology and differentiate between hemorrhagic and ischemic stroke. The CT scanner has the advantage of having equipped CTP machine. Thus performing CTP on these patients confers additional valuable information; with minimal time and cost. Therefore, I wish to evaluate the infarct permeability area on admission perfusion CT as a useful tool in predicting malignant MCA infarction and the need for hemicraniectomy.
Few procedural contraindications exist such as patients with metallic implants, poor renal function, or contrast allergy that is unable to undergo CTP. CTP remains a relatively new and unfamiliar technique to many radiographers. There has also been concern raised that adding CTP to the diagnostic workup may unnecessarily delay thrombolysis.
Potential benefits
Information obtained from this study will help improve decision-making process and improve outcomes in patients with acute ischemic stroke; as well as providing additional valuable information to the existing body of knowledge in this field.
Research objectives
General objectives
The purpose of this study is to investigate the role of CTP in detecting early malignant MCA infarct.
Specific objectives
The following objectives are as follows:
To measure CTP parameters (CBF, CBV, and MTT) in MCA infarct between malignant brain infarct (MBI) and nonMBI. To identify predictive factors for the development of malignant MCA infarct based on clinical condition, CT scan, and CTP parameters. To determine the relationship between CTP parameters (CBF, CBV, and MTT) with a mortality rate at 30 days, patient’s outcome based on Glasgow outcome scale (GOS) on discharge and modified Rankin scale (mRS) at 6 months.
SUBJECTS AND METHODS
Research methodology
Study design
This study is a prospective cohort study which was conducted over a period of 3 years from August 2015 to March 2018 in Hospital Kuala Lumpur. Approval to undertake the project was obtained from the Medical Research Ethics Committee, Ministry of Health.
Study sample
A total of 95 patients with a clinical diagnosis of acute malignant MCA infarct, presenting with symptoms of hemiparesis, sensory or motor deficits, hemianopia, higher cerebral dysfunction dysphasia, aphasia, visuospatial loss at the Department of Neurology and Neurosurgery, Hospital Kuala Lumpur were included in this study. Patients were examined on admission, and a detailed clinical history along with vascular risk profile assessment, handedness, and neurological status including the NIHSS (refer Appendix A) was performed and documented. Routine blood investigations were taken which include full blood count, electrolytes, coagulation profile, and group cross and match in preparation for potential intervention as well as to ensure the renal profile is normal. In view of the CTP scanning that requires contrast administration, the renal profile needed to be normal and patients with history of allergy (except contrast allergy) were given intravenous hydrocortisone 100 mg or prednisolone 40 mg.
Inclusion criteria
The inclusion criteria in this study were patients of (1) age 18–90 years old (2) presentation of acute malignant MCA infarcts such as hemiparesis, sensory or motor deficits, hemianopia, higher cerebral dysfunction dysphasia, aphasia, and visuospatial loss which occur within 48 h of onset.
Exclusion criteria
The exclusion criteria were (1) concurrent hemorrhagic stroke, (2) concurrent other vascular territory infarction, (3) known allergy to iodinated contrast, and (4) renal dysfunction with estimated glomerular filtration rate (eGRF) <30 ml/min.
Follow-up and outcome measurements
Data collected include the patient’s demographic profile, NIHSS, time from onset of presentation, stroke etiology, and risk factors. CTP parameters (CBF, CBV, and MTT) were collected, and mean value was obtained. The presence of infarcted areas was based on the radiology report which is based on the CT brain done on the day of follow-up CT scan. Outcomes of the patients were assessed based on mortality rate at 30 days, GOS at discharge and mRS at 6 months follow-up. Good outcome was classified as GOS = 1–2 and poor outcome as GOS 3–5.
Sampling size and method
The sample size of this study was calculated based on the previous study by Dittrich, MD, Kloska et al. Jneurol (2008) 255:896–902) the expected sensitivity is 0.95 and expected specificity is 0.72 with an expected prevalence of 0.20. Calculation using sensitivity and specificity formula (Buderer, 1996), where α = 0.05, level of confidence is 95% and desire precision is 0.1. The calculated sample size using sensitivity and specificity formula is 98(both arms). Considering 20% drops from the study, the total required number of patient is 122.
Statistical method
The statistical software we employed was the IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. As most of the variables are numerical, their descriptive analyses are expressed as means and standard deviation.
Pearson’s Chi-square test and Fisher’s exact test were used to determine significant differences in outcome based on mortality rate at 30 days, GOS at discharge and mRS at 6 months between patients of two groups; MBI group and nonMBI group. The GOS was dichotomized as the good outcome (GOS 1–2) and poor outcome (GOS 3–5), and the mRS as the good functional outcome (mRS 0–4) and poor outcome (mRS 5–6).
P < 0.05 is considered statistically significant.
Data collection
A specially-designed data collection form was used to gather information concerning the patient’s demography and the variables mentioned above (refer to Appendix B). The raw data were then translated into Microsoft Excel and then transferred to (SPSS) version 20.0.
CTP scanning protocol and data processing
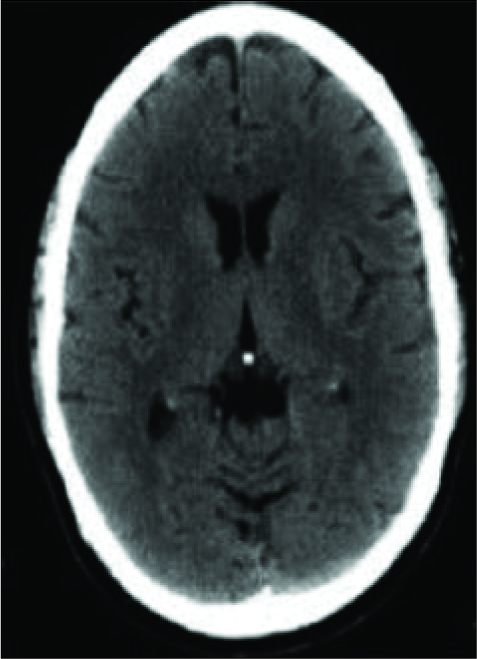
All patients that were admitted to Hospital Kuala Lumpur with acute malignant MCA infarct symptoms such as hemiparesis, sensory or motor deficits, hemianopia, higher cerebral dysfunction dysphasia, aphasia, and visuospatial loss underwent noncontrast CT brain [
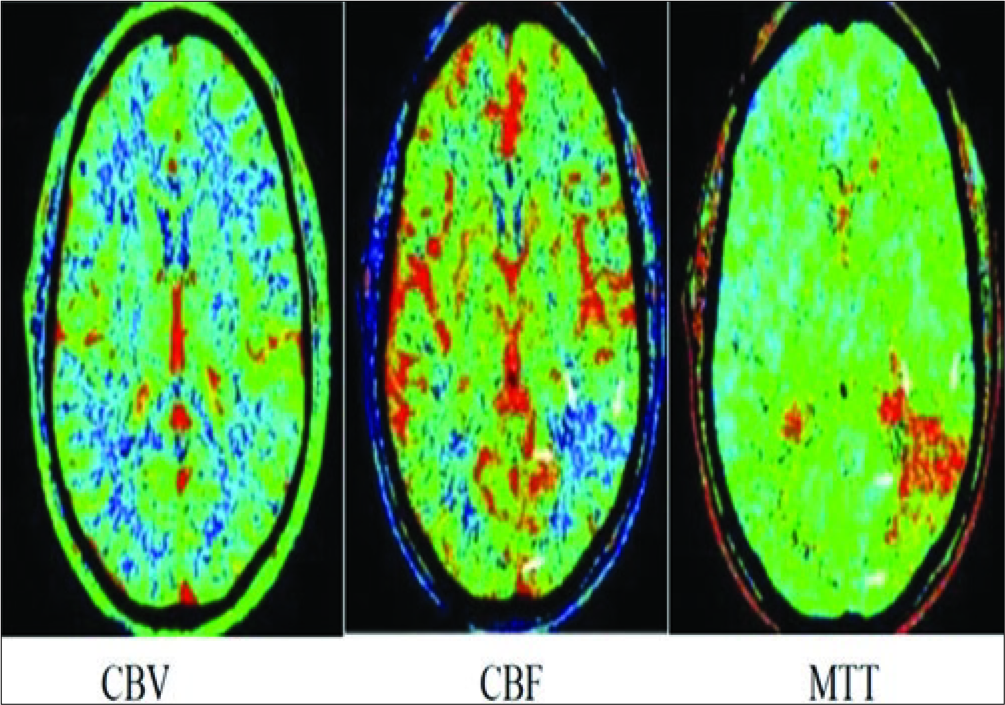
Figure 1b:
Computed tomography perfusion performed 6 hours after onset. Cerebral blood volume( CBV) map showed no abnormality. Cerebral blood flow (CBF) map showed a region of decreased perfusion at the posterior segment of left middle cerebral artery territory( white arrows). Mean transit time showed prolongation within the same region (white arrows).
All examinations were performed on whole brain CTP using a 320-row CT system (Aquilion ONE, Toshiba Medical Systems). Contrast media were infused using the Infusor pump to inject 60 ml of nonionic contrast agent Omnipaque 350 which was infused at the rate of 5 ml/s through a branula of at least size 16G at the antecubital fossa followed by 40 ml of normal saline also at 5 ml/s once the patient is prepared. Power injector was set at a pressure of 300 psi.
The dynamic acquisition protocol, which used a gantry rotation speed of 1 rotation per 5 s acquisition delay allowed acquisition of a mask volume (80 kVp and 200 mAs) without contrast enhancement for subsequent subtraction of the other scans. All subsequent scans were acquired at 200 mAs with continuous scanning during the passage of the contrast medium through the brain. All subsequent scans were acquired at 200 mAs with continuous scanning during the passage of the contrast medium through the brain [refer
Scan protocol
Scan protocol uses plain CT brain by selecting four- dimensional (4D) CT digital subtraction angiography acute stroke CBP. It used plain scan at 5 s postcontrast injection, followed by continuous arterial phase scans started at 7 s postcontrast injection until 22 s. Intermittent phase scans started at 27 s until 64 s with 6 s interval.
Postprocessing
Brain perfusion volume – Vitrea (4D-Vol DYNAMIC 4D CBP), brain perfusion volume to brain analysis CT (Head 0.5 HE) was loaded and 4D perfusion was selected.
The values were then calculated to obtain a mean value for each parameter. The values of interest were the MTT(s), TTP(s), regional CBV (ml/100 ml), and regional CBF (ml/100 g/min). Ischemic core was cytotoxic edema with irreversible ischemia of CBF <10 ml/100 g/min. Penumbra was reversible ischemia with CBF <10–18 ml/100 g/min and CBV that is reduced or normal. Areas that demonstrate matched defects in CBV and MTT represent salvageable infarct core, whereas areas which have prolonged MTT but preserved CBV are considered to be ischemic penumbra [
From the CTP data, normalized color-coded perfusion maps of CBF, CBV, and TTP were calculated using the commercially available software. All CTP investigations were reviewed and evaluated by two neuroradiologists with 10 years experienced.
Patients recruited were grouped into two groups; malignant brain infarction who was indicated for operation and nonMBI which was treated nonsurgically.
Definition of MBI
The patients were divided into those who developed malignant brain infarction versus those who did not. MBI group patients were of: (1) acute, complete MCA infarction with early parenchymal hypodensity of at least 50% of the MCA territory and signs of local brain swelling such as sulcal effacement and compression of the lateral ventricle; (2) midline shift of >5 mm at the septum pellucidum or pineal gland with obliteration of the basal cisterns; and (3) neurological deterioration consisting of a NIHSS increase by >2 points and decrease in the level of consciousness to a score of ≥1 of the NIHSS.
Patients who develop MBI were compared to those without a malignant course. The mean sizes of the ischemic areas generated by the different perfusion maps (i.e., CBF, CBV, and TTP) were compared by means of the t-test between both patients groups for CBF; while CBV and TTP data were skewed, therefore median values were used with Z statistics, and Mann–Whitney U-test was used. To compare sensitivity and specificity, conventional binomial receiver operating characteristics (ROC) analysis was conducted. From the ROC curves, the sensitivity and specificity were calculated. In addition, the positive and negative predictive value for each perfusion map was determined.
RESULTS
Based on
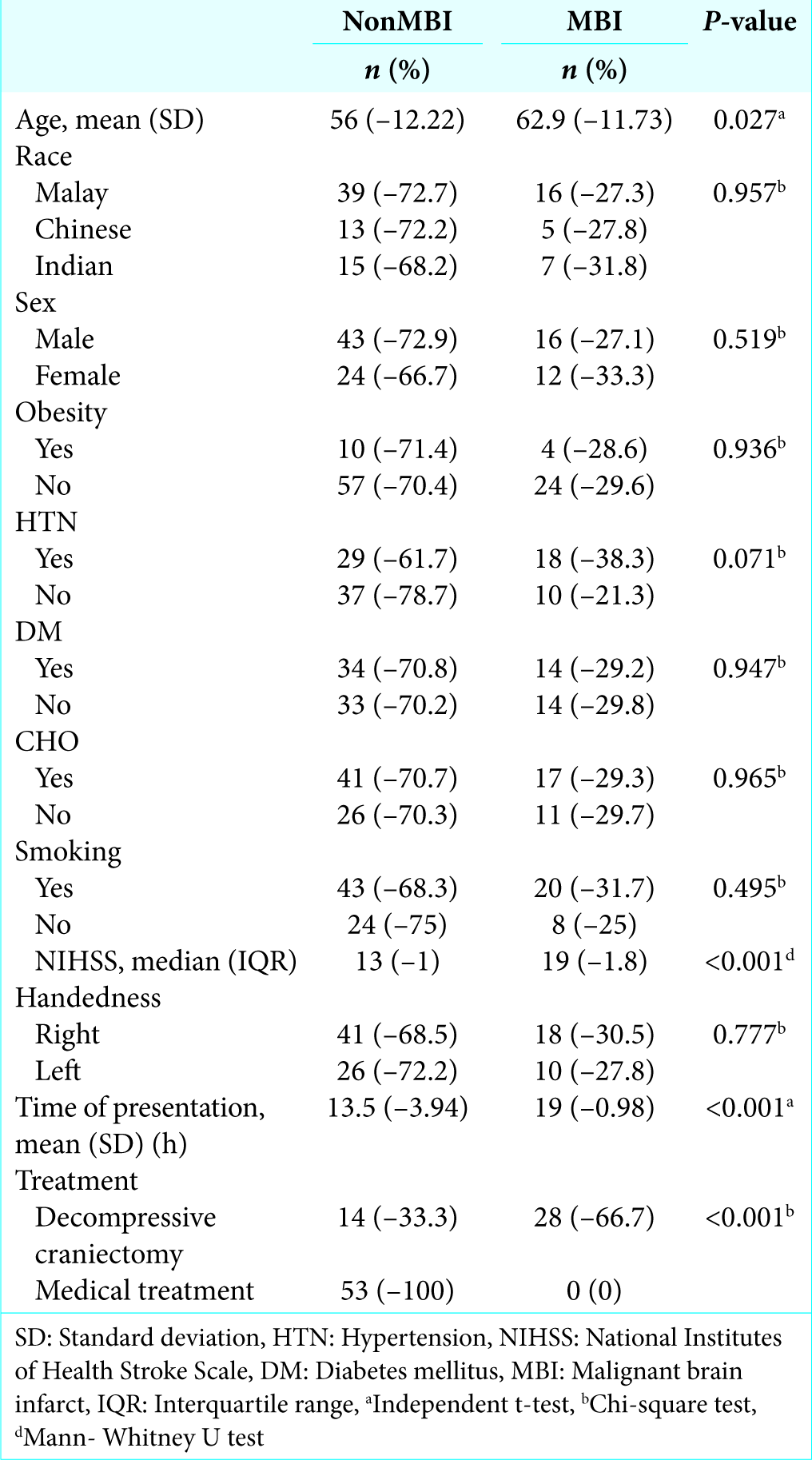
About three-quarter of the study population was male (72.9%) and from Malay ethnicity (72.7%) in nonMBI group compared to (27.1%) male and (27.3%) Malay in MBI group.
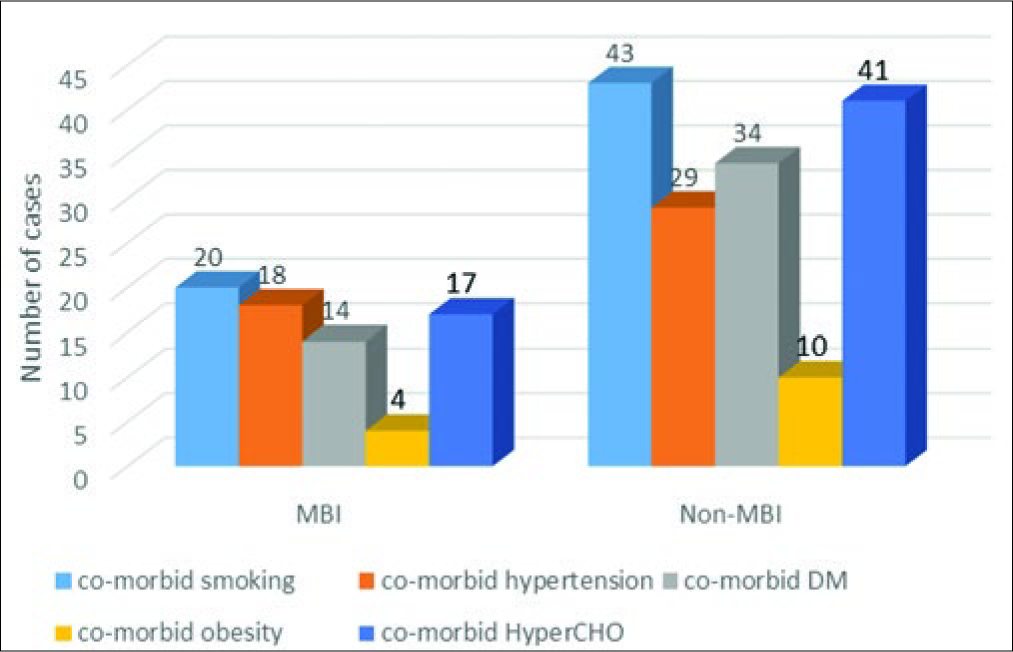
Comorbidities such as obesity, diabetes mellitus (DM), hypercholesterolemia (CHO), smoking, and HTN were documented. Among them, there were lesser patients who had HTN in nonMBI group accounting for 29 patients (61.7%) while higher in MBI group accounting for 18 patients (38.3%) [
On admission, stroke severity as measured by NIHSS was found to be higher in the group with MBI; 19 (1.8%).
Majority of the patients were right-handedness in both malignant (30.5%) and nonMBI groups (68.5%). Time of presentation was longer in the MBI group with a mean (SD) of 19 h and 13.5 h in nonMBI group.
Twenty-eight (66.7%) patients developed MBI, which requires decompressive surgery while 14 (33.3%) patients from nonMBI eventually turned malignant requiring decompressive surgery; as evidence by serial imaging and clinical deterioration.
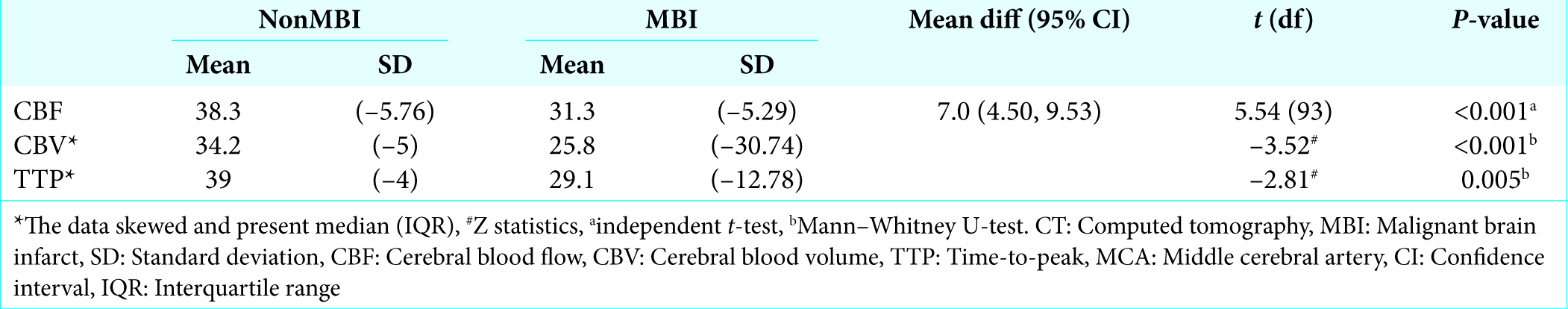
Overall, mean CTP maps were generally larger in patients with nonMBI compared to the MBI. For nonMBI group of patients, results showed CBV with a mean (SD) of 34.2 (5.00) and TTP of 39.0 (4.00) and CBF of 38.3 (5.76), TTP has the highest mean value compared to CBV and CBF. As for MBI group of patients, mean CBV was 25.8 (30.74), TTP of 29.1 (12.78), and CBF of 31.3 (5.29). CBF has the highest mean between the parameters of CTP. This showed that all three parameters of CTP were statistically significant (P < 0.001, 0.005).
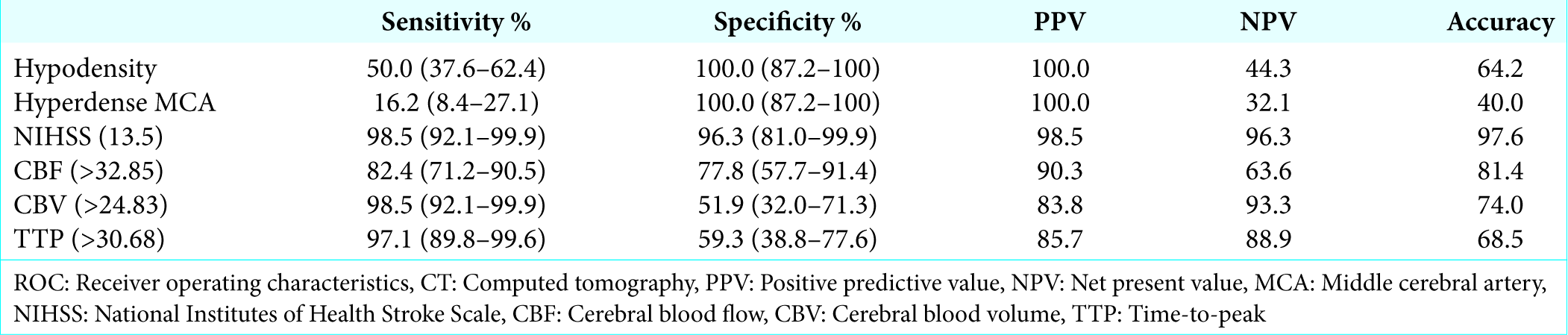
Thirty-four patients showed hypodensity on CT scan, and 11 patients showed a hyperdense MCA sign on the native scan. In the ROC curve analysis for native CT scan, hypodense signs have 50.0 sensitivity and 100.0 specificity while hyperdense MCA sign has a sensitivity of 16.2 and 100.0 specificity.
The most accurate values were obtained for CBF in a mean ROI of >32.85 of the whole hemisphere with a sensitivity of 82.4, a specificity of 77.8, a PPV of 90.3, and a net present value (NPV) of 63.6. The TTP with a cutoff value of >30.68 led to high sensitivity of 97.1 while having low specificity of 59.3, a low PPV of 85.7 and higher NPV of 88.9. For CBV reduction in a mean ROI of >24.83 ROC curve calculations led to a higher sensitivity of 98.5, a lower specificity of 51.9, a similar PPV of 83.8, and a comparable high NPV of 93.3 in predicting a MBI.
The analysis of optimal cutoff NIHSS value which was a stroke severity of NIHSS more than 13.5 on admission showed the highest sensitivity of 98.5 and highest specificity of 96.3 and highest NPV of 98.5 and PPV of 96.3 compared to all CTP parameters.
Comparison of the area under ROC curves between different CTP maps and native CT scan revealed a better prediction of a malignant course. In comparison with CTP maps, NIHSS was almost as accurate to determine malignant brain swelling. There was a clear trend toward better prediction for mean CBF and it has reached the level of significance. Prediction of MBI using all CTP maps was superior to hyperdense MCA sign and hypodensity on native cranial CT scan (mean CBF: P < 0.001, mean CBV: P < 0.001, and mean TTP: P = 0.005).
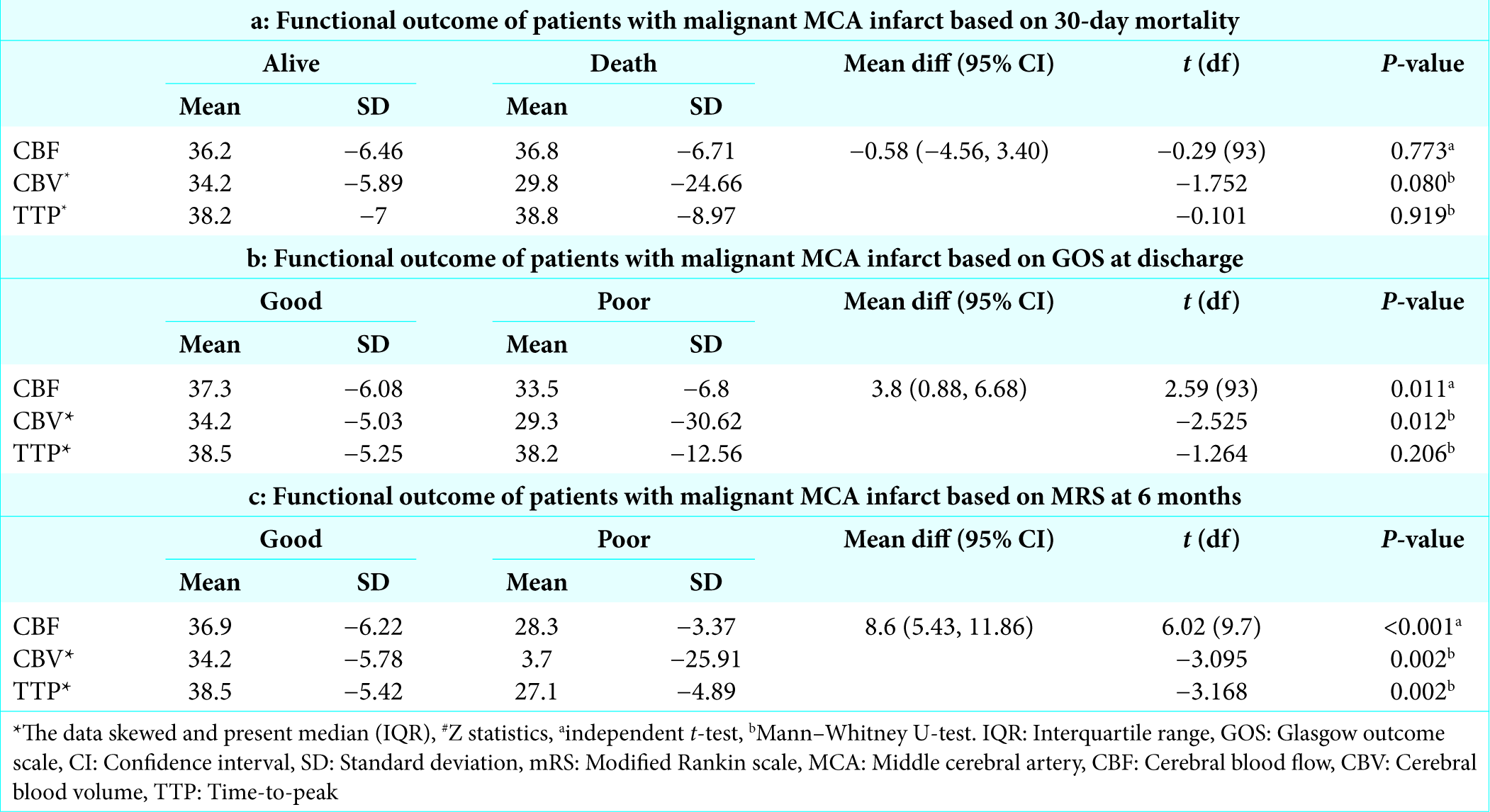
CTP analysis for outcome based on 30-day mortality showed no significant difference in a mean difference of the CTP parameters and mean TTP was higher in both groups of alive group 38.2 (7.00) and death group 38.8 (8.97) [
Functional outcomes on discharge were measured based on GOS where good (GOS 1–2) and poor (GOS 3–5) functional outcome were measured for different CTP parameters; CBF was 37.3 (6.08), CBV was 34.2 (5.03), and TTP was 38.5 (5.25) for good outcome and for poor outcome CBF was 33.5 (6.80), CBV was 29.3 (30.62), and TTP was 38.2 (12.56). CBF and CBV were statistically significant (P = 0.011) and (P = 0.012) [
Clinical condition of patients were also accessed at 6 months on reviewed in neurosurgery clinic using mRS [Appendix C] where good outcome (mRS <4) was seen in all parameters of CTP with mean value ranging from 34.2 to 38.5 for good outcome and mean value for poor outcome ranging from 3.7 to 28.3 with significant results (P = 0.002 and P < 0.001) [
DISCUSSION
Acute stroke remains the leading cause of morbidity and mortality worldwide and accounts for up to 20%–30% of emergency admissions. Among the groups of patients who develop acute stroke, a small subset of 10% of patients, unfortunately, progress to develop malignant MCA infarction, with a reported mortality rate of 80% if left untreated. Decompressive craniectomy is an effective treatment option in reducing morbidity and mortality, as proven by several trials worldwide. Since the publication of the landmark European trials on the effectiveness of decompressive craniectomy in improving the overall outcome of this subset of patients, subsequent trials have raised new questions and fuelled debates particularly on the topic of ideal candidates and timing of surgery. Performing early decompressive craniectomy before the onset of neurological deterioration still remains debatable and not commonly performed due to the uncertainty of edema progression and surgical related complications. Current trends in the research on stroke management emphasizes on the development of predictive tools or models in predicting progression of MBI for clinical decision-making. Previous studies have identified various predictive factors such as NIHSS score, Alberta Stroke Program Early CT score, and diffusion and perfusion MRI imaging to predict the development of malignant brain infarction. In the assessment of acute stroke syndrome, neuroimaging plays a critical role in confirming the diagnosis and determining patient care and outcome. Noncontrast CT scan remains the primary diagnostic modality; being readily available in most if not all centers, fast imaging time and relatively cheaper in cost to acquire and maintain. It, however, lacks the ability to provide crucial information vital to the planning of management in patients with acute stroke, in particular, predicting potential deterioration from space occupying cerebral edema in patients with MCA territory stroke. MRI DWI remains the gold standard in measuring edema volume and predicting high-risk patients; but its limitation in terms of logistics availability, longer acquisition time, requirement of specialized personnel in data interpretation, and higher cost-benefit ratio makes it less than ideal as a principal diagnostic tool of choice in light of urgent decision-making situations.
The role of CTP is rapidly developing and evolving into a diagnostic modality of choice in the management of acute stroke. It has a distinct advantage of relative cost-effectiveness, availability, rapid information acquisition time, and ease of interpretation. CTP allows for accurate identification and differentiation of areas of ischemic penumbra tissue from infarcted tissue; with an additional ability of quantifying CBF. We investigate whether CTP could help in predicting the subsequent development of malignant MCA infarction.
The mean age of patients in this cohort is 56 (SD = 12.22) years old in nonMBI group and 63 (SD = 11.73) in MBI group; the majority of the patients were aged between 30 and 80 years old. Age generally remains a predictor of outcome, in that older patients are protected from the deleterious effects of space occupying edema due to cerebral atrophy resulting in a less malignant course of progression and they deteriorate over a longer time interval. Conversely, younger patients who have not suffered the effects of cerebral atrophy may deteriorate faster, and present with a lower preoperative GCS score. In this study, we did not find an association between age and MBI, possibly due to an increased number of older patients in our study population (54% older than 68 years old). According to Gulensoy et al., as age advances, the result becomes less successful and the mortality increases.[
Male patients (27.1%) were more frequently affected compared to females (33.3%); in contrast to trends reported in literature.[
Patients were stratified based on risk factors for which they play an important role in acute treatment decisions as well as influencing both short-term and long-term outcome in patients. Comorbidities associated with an increased incidence of primary and recurrent strokes such as obesity, DM, smoking, hypercholesterolemia, and HTN were taken into account. Majority of the study population with MBI were hypertensive; 61.7% as compared to 23.2% in the MBI group. The variation of blood pressure and HTN is known risk factors for malignant MCA infarct.[
Clinical parameters remain of upmost importance as the first- line assessment in predicting the development of malignant infarction in patients. Conscious level on admission as well as progressive deterioration of conscious level remains an ominous sign in patients with stroke. The early studies and publication commonly used the GCS scoring system in stroke patients for the purpose of clinical triage and monitoring, primarily due to its reliable interpretation among clinicians. The NIHSS is a reliable, although more detailed score that provides a quantitative measure of stroke-related deficits and is valid in predicting lesion size and measure of stroke severity and important predictor of outcome in stroke patients. In this study, the median NIHSS score among patients with MBI on admission was 19 as compared to 13 in the nonMBI group (P < 0.001). This is similar with previously published papers, elucidating the useful role of NIHSS is in predicting development or progression to MBI in conjunction with other predictors to determine the risk of MBI. An NIHSS >18 was strongly associated with MBI and a NIHSS score at 7 days after admission, of at least 6 accurately forecasts a poor long-term outcome after stroke.[
When compared to clinical parameters, CT findings are less sensitive than the NIHSS in determining early predictors of malignant MCA infarct as clinical presentation of patients still remains the most important indicator compare to radiological results. NIHSS has a sensitivity of 98.5% and specificity of 96.3% in determining predictors of malignant MCA infarct.
Presence of good collateral circulation sustains brain viability to arterial occlusion while poor leptomeningeal collaterals can be ominous of continued tissue damage in the presence of an ischemic insult. Leptomeningeal collateral circulation is critical in maintaining blood flow to the ischemic regions to reduce ischemic injury. Collateral scoring is a useful tool for assessing leptomeningeal collateral circulation on CTA. Assessment of perfusion by collateral flow and ischemic area volume is important; however, it does not show the perfusion state and ischemic core size. Despite its importance, it has been difficult to simply and quantitatively measure the degree of collateral flow. We chose not to include this parameter in our study because this requires CT angiography for collateral scoring.[
This study is aimed at identifying the usefulness of CTP parameters in predicting development or progression of malignant edema in MCA territory infarction. We aimed to identify the scanning parameters best suited to indicate tissue at risk and to measure a perfusion limit to predict infarction and correlating CTP findings with the patient’s outcome based on mRS. CTP based mapping of CBF, CBV, and TTP allowed for the discrimination between patients without a relevant risk and those with considerable risk of developing life-threatening brain swelling and a malignant course of the infarct.
Our results show a significant difference between perfusion parameters [
The CBF and TTP maps describe the extent of the hypoperfusion lesion, whereas the most profoundly affected regions can be shown as a decrease in CBV. Cerebral autoregulation can be diagnosed when there is an increased in CBF and TTP but no difference in CBV.[
CTP is able to assess the microcirculation and distal cerebral vessels; it is not operator dependent and reproducible in addition to providing details of the CBF, CBV, and TTP that may be used to determine the areas of MCA infarct. CTP is an easy, readily available and has a fast acquisition time, especially for critically ill patients with reproducible results. It was possible to differentiate between infarcted tissue (infarcted core) with viable tissue (penumbra) using CT parameters by absolute CBF and CBV values and in larger series of 130 patients, by relative TTP delay and absolute reduction of CBV.[
Before the use CTP as a potential tool for predicting malignant brain edema, several signs were indicative as early markers of malignant MCA infarction on plain CT brain, being the primary diagnostic modality in all cases of acute stroke. Among the signs of identification for malignant MCA infarct, the most ominous are hyperdense MCA sign, signifying large MCA trunk occlusion and are found to be related to a fatal outcome. Therefore, close observation of the clinical condition of patients and measures must be taken to prevent worsening of neurology.
In this series, when comparing sensitivity and specificity of imaging parameters between plain CT and CTP parameters in a cohort of patients with MCA territory infarction [
Between CTP parameters, reduction in CBF values had high sensitivity and specificity rate for the development of MBI; 82.4% and 77.8%, respectively. CBV was highly sensitive (98.5) in detecting potential progression or development of MBI but lack specificity (51.9); with higher negative predictive value at 93.3%. TTP values exhibited a similar rate of high sensitivity and low specificity (97.1% and 59.3%, respectively) with comparable
Similar accounts can be attributed to a high sensitivity value of TTP, which relates to penumbra region reperfusion in cases of ischemia-infarction. Thus, when interpreting values of CTP parameters, a reduction of CBV is a sensitive indicator of the progression of malignant brain infarction, while a global reduction of CBF is both sensitive and specific to the development of malignant brain infarction. CTP ability to depicts the extent of ischemia tissue indirectly helps to predict the patients’ clinical outcome. The highest sensitivity in depicting ischemic tissue was achieved by mapping the CBV (98.5%) and TTP (97.1%) while CBF were found to be of moderate specificity (77.8%) with moderate sensitivity (82.4%).
MBI portends poor outcome and high mortality rate as are seen in numerous prior publications. Decompressive craniectomy performed within 24–48; and in a few small studies, up to 96 h significantly reduce mortality in patients severe ischemic brain edema.[
The 30-day mortality of malignant MCA infarct is up to 25% in the MBI group while 6% in nonMBI in our study. The mortality was considerably higher than that of 15% reported by Ng and Mimmannitya possibly due to a high incidence of malignant MCA infarct affecting older patients in our group.[
The question of hemispheric dominance and outcome in stroke remains under constant debate. Key to the argument stems from the measures of outcome used, common in all cases is the Rankins’ score system. The Rankins’ score system has been criticized as primarily focusing predominantly on motor function outcome, and in such case, cognitive function assessment is not given the required weightage in the overall outcome and functional recovery. Despite the ongoing debate and variation in published outcomes among patients with dominant or nondominant malignant MCA infarction, it is generally agreed that the results are better when surgery is performed on the nondominant hemisphere in most series. Patients with an infarct in the dominant hemisphere may have a lower quality of life than patients with an infarct in the nondominant hemisphere because they have more severe language impairment. However, a meta-analysis found no difference in functional outcome between right and left hemispheric infarcts.[
CONCLUSION
In summary, objective and comprehensive predictive models require both clinical and radiological parameters to assist in rapid and safe clinical decision making in acute stroke patients. This study shows that among the various published predictive markers, CT perfusion parameters of TTP & CBF values; in combination with severity of clinical presentation using the NIHSS score system is sensitive and specific in predicting development of malignant brain infarction in patients presenting with acute stroke. This may potentially be used as a relevant and effective bedside assessment tool for rapid and safe decision making process.
References
1. Bang OY, Lee PH, Heo KG, Joo US, Yoon SR, Kim SY. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J Neurol Neurosurg Psychiatry. 2005. 76: 1222-8
2. Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying (“malignant”) middle cerebral artery infarction under conservative intensive care. Intensive Care Med. 1998. 24: 620-3
3. Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997. 40: 1168-75
4. Cho DY, Chen TC, Lee HC. Ultra-early decompressive craniectomy for malignant middle cerebral artery infarction. Surg Neurol. 2003. 60: 227-32
5. Clarke DJ, Forster A. Improving post-stroke recovery: The role of the multidisciplinary health care team. J Multidiscip Healthc. 2015. 8: 433-42
6. Demchuk AM, Wein TH, Felberg RA, Christou I, Alexandrov AV. Evolution of rapid middle cerebral artery recanalization during intravenous thrombolysis for acute ischemic stroke. Circulation. 1999. 100: 2282-3
7. Foerch C, Otto B, Singer OC, Neumann-Haefelin T, Yan B, Berkefeld J. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. 2004. 35: 2160-4
8. Gulensoy B, Karatay M, Erdem Y, Celik H, Tascioglu T, Sertbas I. Decompressive hemicraniectomy for malignant middle cerebral artery infarct. Turk Neurosurg. 2016. 26: 704-8
9. Gupta R, Connolly ES, Mayer S, Elkind MS. Hemicraniectomy for massive middle cerebral artery territory infarction: A systematic review. Stroke. 2004. 35: 539-43
10. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. “Malignant” middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996. 53: 309-15
11. Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998. 50: 341-50
12. Hofmeijer J, Amelink GJ, Algra A, van Gijn J, Macleod MR, Kappelle LJ. Hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial (HAMLET). Protocol for a randomised controlled trial of decompressive surgery in space-occupying hemispheric infarction. Trials. 2006. 7: 29-
13. Hotter B, Ostwaldt AC, Levichev-Connolly A, Rozanski M, Audebert HJ, Fiebach JB. Natural course of total mismatch and predictors for tissue infarction. Neurology. 2015. 85: 770-5
14. Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS One. 2017. 12: e0171425-
15. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized, controlled trial. Stroke. 2007. 38: 2518-25
16. Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014. 370: 1091-100
17. Kasner SE, Cucchiara BL, McGarvey ML, Luciano JM, Liebeskind DS, Chalela JA. Modified national institutes of health stroke scale can be estimated from medical records. Stroke. 2003. 34: 568-70
18. Koh MS, Goh KY, Tung MY, Chan C. Is decompressive craniectomy for acute cerebral infarction of any benefit. ? Surg Neurol. 2000. 53: 225-30
19. Kumar A, Sharma MS, Sharma BS, Bhatia R, Singh M, Garg A. Outcome after decompressive craniectomy in patients with dominant middle cerebral artery infarction: A preliminary report. Ann Indian Acad Neurol. 2013. 16: 509-15
20. Leonhardt G, Wilhelm H, Doerfler A, Ehrenfeld CE, Schoch B, Rauhut F. Clinical outcome and neuropsychological deficits after right decompressive hemicraniectomy in MCA infarction. J Neurol. 2002. 249: 1433-40
21. Lin L, Bivard A, Parsons MW. Perfusion patterns of ischemic stroke on computed tomography perfusion. J Stroke. 2013. 15: 164-73
22. Manno EM, Rabinstein AR, Wijdicks EF.editors. The acute and chronic management of large cerebral infarcts. Intensive Care Medicine. Yearbook of Intensive Care and Emergency Medicine. Vol. 2007. Berlin, Heidelberg: Springer; p.
23. Mayer SA. Hemicraniectomy: A second chance on life for patients with space-occupying MCA infarction. Stroke. 2007. 38: 2410-2
24. Ng LK, Nimmannitya J. Massive cerebral infarction with severe brain swelling: A clinicopathological study. Stroke. 1970. 1: 158-63
25. Oppenheim C, Samson Y, Manaï R, Lalam T, Vandamme X, Crozier S. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000. 31: 2175-81
26. Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MF. Timing of neurologic deterioration in massive middle cerebral artery infarction: A multicenter review. Crit Care Med. 2003. 31: 272-7
27. Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V. Decompressive surgery in space-occupying hemispheric infarction: Results of an open, prospective trial. Crit Care Med. 1995. 23: 1576-87
28. Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009. 72: 2104-10
29. Tateyama K, Kobayashi S, Murai Y, Teramoto A. Assessment of cerebral circulation in the acute phase of subarachnoid hemorrhage using perfusion computed tomography. J Nippon Med Sch. 2013. 80: 110-8
30. Thomalla GJ, Kucinski T, Schoder V, Fiehler J, Knab R, Zeumer H. Prediction of malignant middle cerebral artery infarction by early perfusion and diffusion-weighted magnetic resonance imaging. Stroke. 2003. 34: 1892-9
31. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007. 6: 215-22
32. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke. 2007. 38: 2506-17
33. von Kummer R. MRI: The new gold standard for detecting brain hemorrhage?. Stroke. 2002. 33: 1748-9
34. Wartenberg KE. Malignant middle cerebral artery infarction. Curr Opin Crit Care. 2012. 18: 152-63
35. Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003. 34: 1951-6
36. Wintermark M, Reichhart M, Cuisenaire O, Maeder P, Thiran JP, Schnyder P. Comparison of admission perfusion computed tomography and qualitative diffusion and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke. 2002. 33: 2025-31
37. Yundt KD, Grubb RL, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998. 18: 419-24