- Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan.
- Department of Cardiac Surgery, The University of Tokyo Hospital, Tokyo, Japan.
Correspondence Address:
Satoshi Koizumi, Department of Neurosurgery, The University of Tokyo Hospital, Tokyo, Japan.
DOI:10.25259/SNI_1101_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daisuke Sato1, Satoshi Koizumi1, Motoyuki Umekawa1, Satoshi Kioyofuji1, Masahiko Ando2, Minoru Ono2, Nobuhito Saito1. Controversies and challenges of coil embolization for intracranial aneurysm in a continuous-flow LVAD implanted patient: A case report. 27-Jan-2023;14:34
How to cite this URL: Daisuke Sato1, Satoshi Koizumi1, Motoyuki Umekawa1, Satoshi Kioyofuji1, Masahiko Ando2, Minoru Ono2, Nobuhito Saito1. Controversies and challenges of coil embolization for intracranial aneurysm in a continuous-flow LVAD implanted patient: A case report. 27-Jan-2023;14:34. Available from: https://surgicalneurologyint.com/surgicalint-articles/12122/
Abstract
Background: Continuous-flow left ventricular assist device (CF-LVAD) technology has rapidly developed to support the failing heart refractory to standard medical treatments. Although the expected prognosis has improved dramatically, ischemic and hemorrhagic strokes are possible complications and the leading causes of death in the CF-LVAD population.
Case Description: We encountered a case of an unruptured large internal carotid aneurysm in a patient with a CF-LVAD. Following a detailed discussion of his expected prognosis, the risk of aneurysm rupture, and the inherited risk of aneurysm treatment, coil embolization was performed without adverse events. The patient remained recurrence-free for 2-year postoperatively.
Conclusion: This report illustrates the feasibility of coil embolization in a CF-LVAD recipient and emphasizes the necessity of vigilant consideration of whether to intervene in an intracranial aneurysm after CF-LVAD implantation. We confronted several challenges during the treatment: optimal endovascular technique, management of antithrombotic drugs, safe arterial access, desirable perioperative imaging modalities, and prevention of ischemic complications. This study aimed to share this experience.
Keywords: Coil embolization, Intracranial aneurysm, Left ventricular assist device
INTRODUCTION
Continuous-flow left ventricular assist device (CF-LVAD) is a mechanical circulatory support device for patients with severe heart failure and unresponsive to standard pharmacological therapy.[
We encountered a patient with an incidentally detected large internal carotid artery aneurysm after CF-LVAD implantation. The patient was on the waitlist for heart transplantation, and as compared to Western countries, the expected waitlist time in Japan is significantly longer, now 6–8 years. Such a long waitlist and an improved prognosis for CF-LVAD-implanted or eventually transplanted patients have led us to discuss whether to follow-up on this aneurysm conservatively or undergo surgical procedures. Transarterial embolization of intracranial mycotic aneurysms after CF-LVAD implantation has been previously reported.[
CASE ILLUSTRATION
A 46-year-old man with a history of CF-LVAD implantation for fulminant cardiomyopathy 5 years ago [
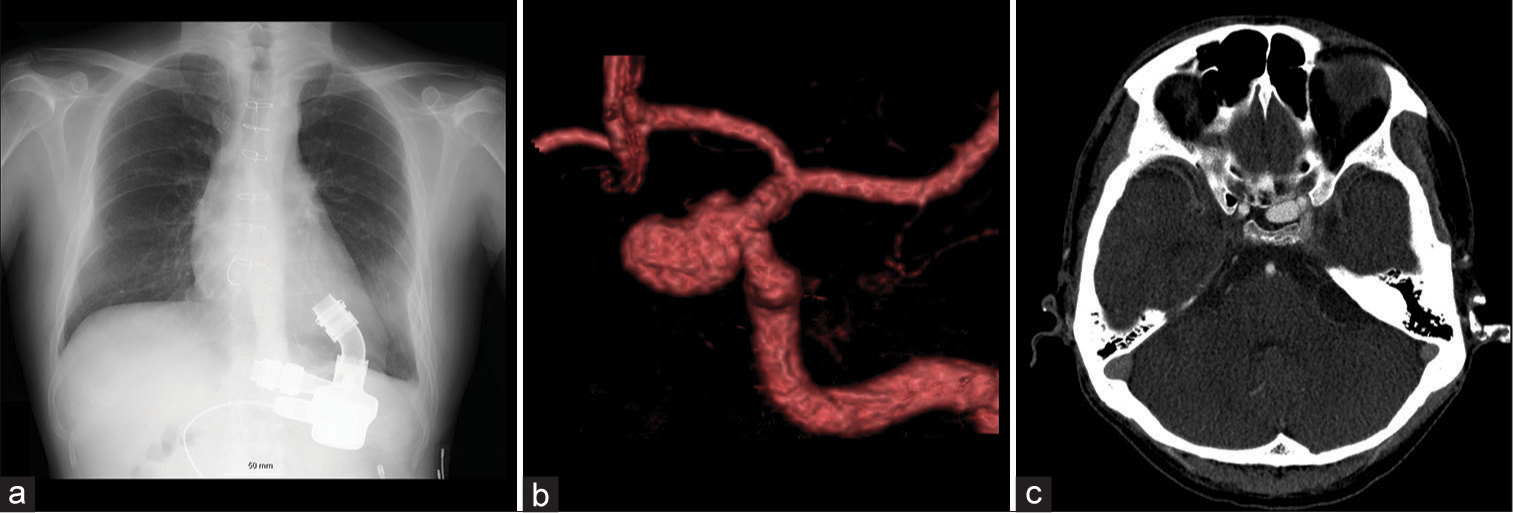
Figure 1:
Continuous-flow left ventricular assist device (CF-LVAD) was implanted for fulminant cardiomyopathy (a, chest X-ray). A large aneurysm was incidentally found at the C2 portion of the left internal carotid artery, 13 mm in its size, and had a bilobular shape (b, CTA). The aneurysm was projected medially and situated at the sellar region (c, CTA). CTA: Computed tomography angiography, LVAD: Left ventricular assist device.
Endovascular treatment
The procedure was performed under general anesthesia. Both aspirin and warfarin were continued during the periprocedural period. A 6-Fr FUBUKI Dilator Kit (ASAHI INTECC, Aichi, Japan) was inserted into the right femoral artery under ultrasound guidance. Since pulsation was weak due to multiple punctures for cardiovascular intervention and the continuous flow created by the CF-LVAD, ultrasound guidance was necessary for accurate puncture. Following the femoral artery puncture, 5000 U of heparin was injected, and the tip of the guiding catheter was perfused with heparin-added saline to control the activated clotting time between 200 and 250 min. Although the patient was a CF-LVAD recipient, there were no access route difficulties. After advancing the guide catheter to the left internal carotid artery, the aneurysm was confirmed by angiography [
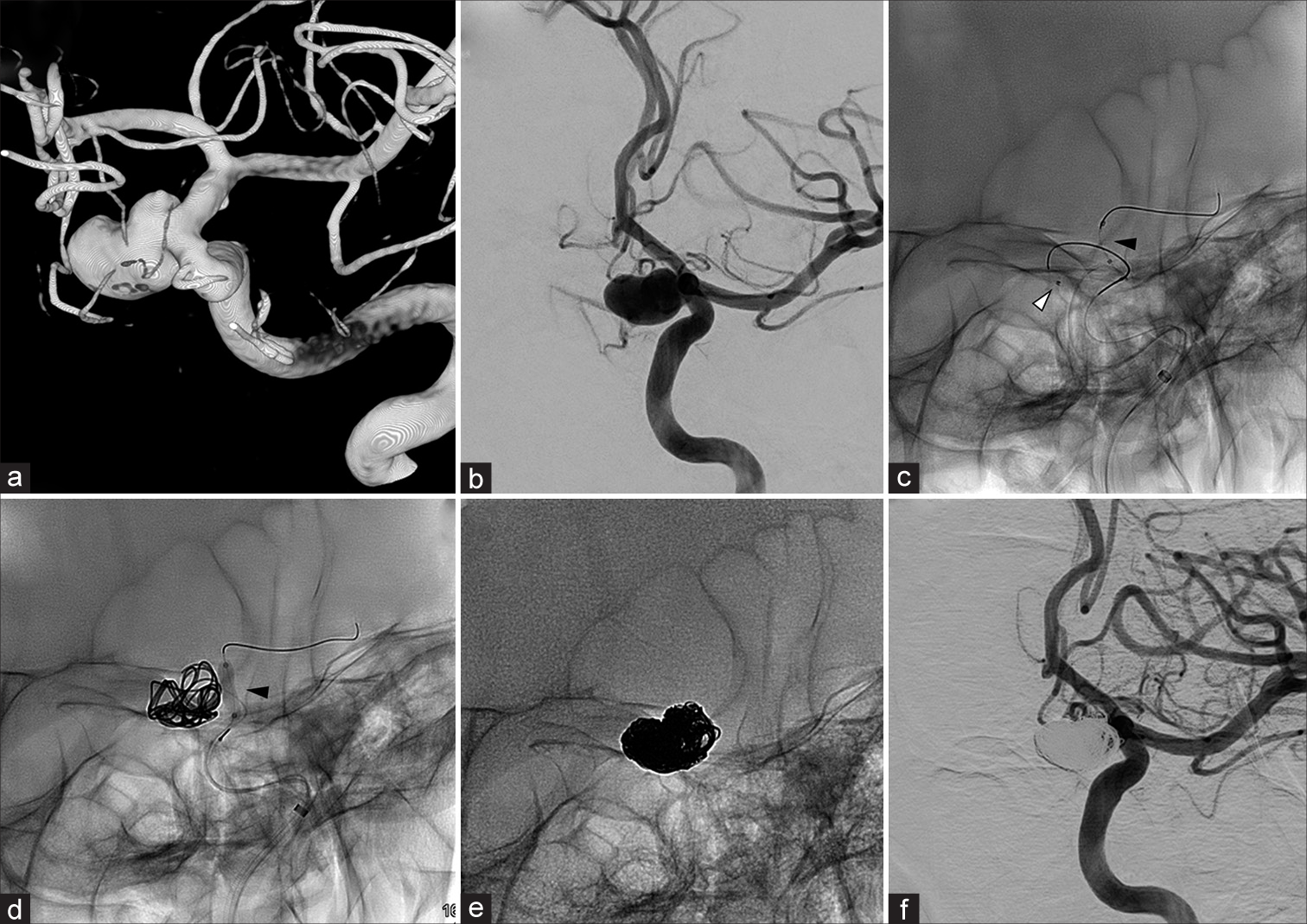
Figure 2:
The aneurysm was confirmed by cerebral angiography (a and b). A 3.4-F TACTICS (Technocrat Corporation, Aichi, Japan) and Headway-17 (TERUMO, Tokyo, Japan) were coaxially inserted, and the microcatheter was placed inside the aneurysm (c; black arrowhead, balloon catheter; white arrowhead, the tip of microcatheter). Shouryu SR 4*10 (Kaneka, Osaka, Japan) was used as a balloonremodeling technique (d; black arrowhead, balloon catheter). Eighteen coils were inserted (e). The contrast of aneurysm has almost disappeared on injection (body filling: f).
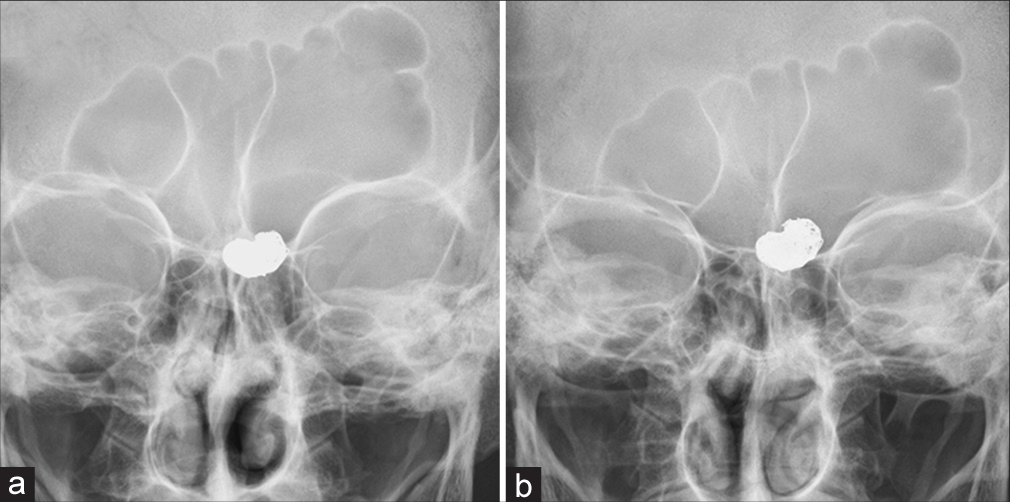
Since magnetic resonance imaging (MRI) did not apply to patients with CF-LVAD implantation, we followed the patient with CT and head X-ray scans for a year to date. Routine CT angiography with contrast material was waived because the patient had moderate chronic kidney disease. Plain CT tomography revealed no ischemic or hemorrhagic strokes. Even though the head X-ray scan performed 1 year after the treatment showed a minimal conformational change in the coil [
DISCUSSION
The 6-month survival rate of patients with severe heart failure was approximately 50% in the 20th century.[
We encountered several challenges during the treatment. The aneurysm had a wide neck and was large, which necessitated adjunctive techniques such as the stent-assisted technique or the balloon-remodeling technique. The placement of the flow diverter stent was also considered. However, the patient was already taking aspirin and warfarin on a daily basis to prevent pump thrombosis. Dual antiplatelet therapy is usually administered during the perioperative period of stent-assisted coiling and flow diverter stenting to prevent in-stent thrombosis.[
Physicians should also recognize that patients with CF-LVAD often have a weak pulse since arterial blood flow is nonpulsatile due to CF-LVAD support, not a physiologically pulsatile pulse as in regular patients. Furthermore, patients often have a history of several punctures, such as percutaneous coronary intervention, intra-aortic balloon pumping, and percutaneous cardiopulmonary support, during the treatment of heart failure. This can also result in a weak pulse. Neurosurgeons should not hesitate to use US guidance.[
The perioperative imaging modality is another pitfall since MRI does not apply to patients with CF-LVAD. Physicians should consider whether an MRI is necessary and how to follow-up with the patient. We evaluated the patient without the aid of MRI using radiography, CT, or cerebral angiography.
Finally, caution must be exercised regarding ischemic complications. Pump thrombosis is a feared complication of CF-LVAD, and strict control of the PT-INR is recommended.[
CONCLUSION
A patient with a CF-LVAD underwent coil embolization for an unruptured intracranial aneurysm without any complications. Controversies and challenges regarding treatment have been demonstrated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012. 125: 3191-200
2. Bender MT, Vo CD, Jiang B, Campos JK, Zarrin DA, Xu R. Pipeline embolization for salvage treatment of previously stented residual and recurrent cerebral aneurysms. Interv Neurol. 2018. 7: 359-69
3. Birati EY, Rame JE. Diagnosis and management of LVAD thrombosis. Curr Treat Options Cardiovasc Med. 2015. 17: 361
4. Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The flolan international randomized survival trial (FIRST). Am Heart J. 1997. 134: 44-54
5. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013. 381: 1107-15
6. Frontera JA, Starling R, Cho SM, Nowacki AS, Uchino K, Hussain MS. Risk factors, mortality, and timing of ischemic and hemorrhagic stroke with left ventricular assist devices. J Heart Lung Transplant. 2017. 36: 673-83
7. Fukushima N, Ono M, Saiki Y, Sawa Y, Nunoda S, Isobe M. Registry report on heart transplantation in Japan (June 2016). Circ J. 2017. 81: 298-303
8. Funatsu T, Ishikawa T, Yamaguchi K, Eguchi S, Matsuoka G, Moriya K. Intracranial mycotic aneurysm after left ventricular assist device implantation treated with trans-arterial embolization via the brachial artery: A case report. NMC Case Rep J. 2021. 8: 433-8
9. Gwyn JC. Left ventricular assist devices. J Intensive Care Soc. 2020. 21: 355-8
10. Hwang JH, Park SW, Kwon YW, Min J, Chee HK, Shin JK. Ultrasonography-guided antegrade common femoral artery approach: Factors associated with access time. J Vasc Access. 2021. 22: 364-9
11. Investigators UJ, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
12. Kawamura S, Koizumi S, Umekawa M, Miyawaki S, Kinoshita O, Ono M. Long-term benefit of mechanical thrombectomy for acute ischemic stroke in patients with a left ventricular assist device: A single-center retrospective study. World Neurosurg. 2022. 165: e331-6
13. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015. 34: 1495-504
14. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017. 36: 1080-6
15. Lalonde SD, Alba AC, Rigobon A, Ross HJ, Delgado DH, Billia F. Clinical differences between continuous flow ventricular assist devices: A comparison between HeartMate II and HeartWare HVAD. J Card Surg. 2013. 28: 604-10
16. Long B, Robertson J, Koyfman A, Brady W. Left ventricular assist devices and their complications: A review for emergency clinicians. Am J Emerg Med. 2019. 37: 1562-70
17. Ma Y, Zhang X, Zhang T, Feng Y, Zhao W, Chen X. Safety and efficacy of dual antiplatelet therapy combining aspirin and ticagrelor in patients with undergoing intracranial stenting procedures. J Neurosurg Sci. 2022. p. Online ahead of print
18. Mehra MR, Cleveland JC, Uriel N, Cowger JA, Hall S, Horstmanshof D. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: A study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail. 2021. 23: 1392-400
19. Mehra MR, Gustafsson F. Left ventricular assist devices at the crossroad of innovation in advanced heart failure. J Card Fail. 2021. 27: 1291-4
20. Park SJ, Milano CA, Tatooles AJ, Rogers JG, Adamson RM, Steidley DE. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012. 5: 241-8
21. Satow T, Ikeda G, Takahashi JC, Iihara K, Sakai N. Coil embolization for unruptured intracranial aneurysms at the dawn of stent era: Results of the Japanese registry of neuroendovascular therapy (JR-NET) 3. Neurol Med Chir (Tokyo). 2020. 60: 55-65
22. Siddiqui AH, Monteiro A, Hanel RA, Kan P, Mohanty A, Cortez GM. Triple therapy versus dual-antiplatelet therapy for dolichoectatic vertebrobasilar fusiform aneurysms treated with flow diverters. J Neurointerv Surg. 2022. p. neurintsurg-2022-019151 Online ahead of print
23. Suarez-Pierre A, Lui C, Zhou X, Giuliano K, Etchill E, Almaraz-Espinoza A. Long-term survival after heart transplantation: A population-based nested case-control study. Ann Thorac Surg. 2021. 111: 889-98
24. Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J. Registry of the international society for heart and lung transplantation: Twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007. 26: 769-81
25. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ. Heart disease and stroke statistics-2016 update: A report From the American heart association. Circulation. 2016. 133: e38-360