- Department of Neurosurgery, University of California, Los Angeles, Los Angeles, California, USA
- Department of Pathology, University of California, Los Angeles, Los Angeles, California, USA
- Jonsson Comprehensive Cancer Center, University of California, Los Angeles, Los Angeles, California, USA
- Department of Neurosurgery, Yeungnam University College of Medicine, Daemyung-dong, Nam-gu, Daegu, Korea
Correspondence Address:
Isaac Yang
Department of Neurosurgery, University of California, Los Angeles, Los Angeles, California, USA
Jonsson Comprehensive Cancer Center, University of California, Los Angeles, Los Angeles, California, USA
DOI:10.4103/sni.sni_423_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Seung J. Lee, Minsu Kim, Carlito Lagman, Timothy T. Bui, William H. Yong, Isaac Yang. Corpora amylacea mimicking low-grade glioma and manifesting as a seizure: Case report. 26-Apr-2017;8:64
How to cite this URL: Seung J. Lee, Minsu Kim, Carlito Lagman, Timothy T. Bui, William H. Yong, Isaac Yang. Corpora amylacea mimicking low-grade glioma and manifesting as a seizure: Case report. 26-Apr-2017;8:64. Available from: http://surgicalneurologyint.com/surgicalint-articles/corpora-amylacea-mimicking-low%e2%80%91grade-glioma-and-manifesting-as-a-seizure-case-report/
Abstract
Background:Corpora amylacea (CA) are accumulations of polyglucosan bodies typically found in astrocytic foot processes, and rarely, can mimic neoplasm. CA accumulation has also been associated with seizure disorders. We report the first case of a histologically confirmed intracranial, intraparenchymal CA lesion mimicking a low-grade glioma and manifesting as a seizure.
Case Description:A 43-year-old man presented after a general tonic–clonic (GTC) seizure. Brain magnetic resonance imaging (MRI) revealed a small lesion in the right mesial temporal lobe with radiologic features of a low-grade glioma. The patient underwent a right pteronial craniotomy for resection of the lesion. Histology demonstrated abundant polyglucosan bodies without neoplastic features. The patient tolerated the procedure well, was free from seizures without antiepileptic drugs at 2-week follow-up, and is undergoing serial surveillance.
Conclusion:The clinical manifestation of CA as a seizure in the context of an identified brain mass is extraordinarily rare. Nevertheless, CA should be considered in the differential diagnosis for patients with seizures and a radiologically identifiable low-grade lesion. Symptomatic CA lesions Mimicking a low-grade glioma should be surgically pursued with a goal of safe, maximal resection to confirm the diagnosis and to provide the patient with prognosis, which can significantly impact patient quality of life.
Keywords: Case reports, corpora amylacea, glioma, pathology, seizures
INTRODUCTION
Corpora amylacea (CA) are aggregates of polyglucosan bodies consisting of insoluble polysaccharides and protein.[
CASE DESCRIPTION
History and examination
A 43-year-old, right-handed man without a prior history of epilepsy presented to his neurologist for evaluation of a GTC seizure. He had no other symptoms and was neurologically intact. He was prescribed an antiepileptic drug and underwent radiological evaluation.
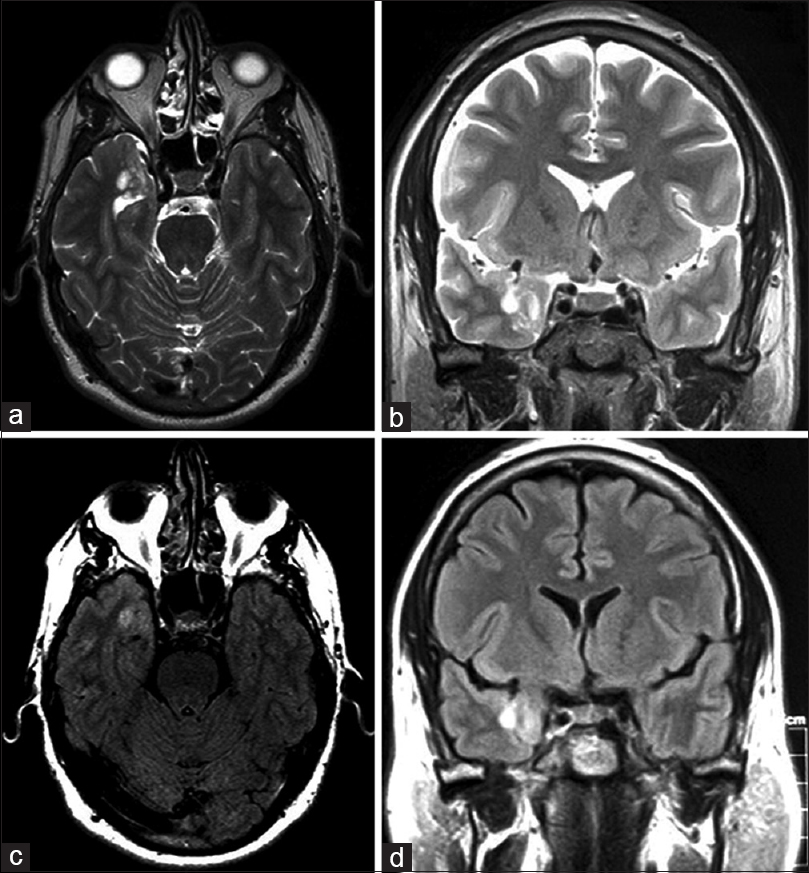
Magnetic resonance imaging (MRI) of the brain revealed a nonenhancing lesion measuring 1.5 × 1.8 cm in the right mesial temporal lobe with minimal mass effect. The lesion appeared hyperintense on T2-weighted images [Figure
A right pteronial craniotomy was performed for resection of the mass. The lesion was noted to have a firm consistency, ill-defined planes, and significant pial adhesions. The lesion was meticulously resected under the operative microscope, and the specimen was sent to pathologic examination. The patient tolerated the procedure well. Immediate postoperative imaging demonstrated confirmed gross-total resection without evidence of ischemia or abnormal enhancement. At 2-week follow-up, the patient was free from seizures and no longer required antiepileptic drugs. The patient has not returned in the 13 months since the surgery.
Pathological examination
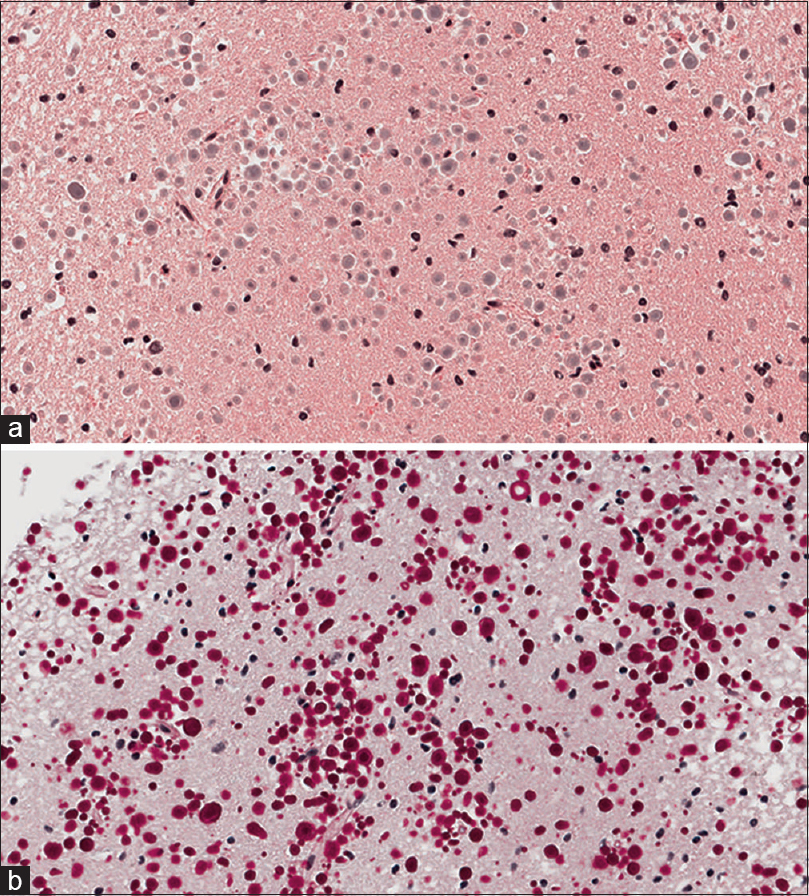
Histological examination of the lesion revealed normal gray matter with focally prominent polyglucosan body accumulations in the brain parenchyma. Numerous spherical polyglucosan bodies were identified on Hematoxylin and Eosin (H and E) frozen section slides without features of neoplasia. Permanent section H and E slides [
DISCUSSION
Glucose monomers within neuroglial cells are thought to polymerize in the setting of cellular degeneration to form aggregates of polyglucosan bodies (hence the term corpora amalycea, Latin for body and starchy, respectively); however, the physiologic and pathologic significance of these lesions are not fully elucidated.[
CA accumulation can also have a genetic etiology. Adult polyglucosan body disease (APBD) is a rare autosomal recessive genetic disorder caused by a glycogen branching enzyme deficiency, leading to CA accumulation in the central and peripheral nervous system.[
MRI is highly sensitive for brain tumors, although it lacks the specificity to distinguish low-grade from high-grade neoplasms.[
PET imaging has also been proven to be useful in the diagnosis of low-grade gliomas. Chen et al. reported that the sensitivity in detecting brain tumors with 18F-DOPA is higher (sensitivity, 96%; 95% CI, 87–100%) than 18F-fludeoxyglucose (18F-FDG) PET imaging (sensitivity, 61%; 95% CI, 41–81%).[
CA accumulation has been described in patients with temporal lobe epilepsy and other seizure disorders.[
Abel et al.[
CONCLUSION
This case illustrates an extraordinarily rare instance in which CA aggregates appeared as a well-demarcated, spherical mass lesion causing a seizure. Although uncommon, CA should be considered in the differential diagnosis for lesions with radiological characteristics of a low-grade glioma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abel TJ, Hebb AO, Keene CD, Born DE, Silbergeld DL. Parahippocampal corpora amylacea: Case report. Neurosurgery. 2010. 66: E1206-7
2. Bigio EH, Weiner MF, Bonte FJ, White CL. Familial dementia due to adult polyglucosan body disease. Clin Neuropathol. 1997. 16: 227-34
3. Busard HL, Gabreels-Festen AA, Renier WO, Gabreels FJ, Joosten EM, van ‘t Hof MA. Adult polyglucosan body disease: The diagnostic value of axilla skin biopsy. Ann Neurol. 1991. 29: 448-51
4. Cavanagh JB. Corpora-amylacea and the family of polyglucosan diseases. Brain Res Brain Res Rev. 1999. 29: 265-95
5. Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W. 18F-FDOPA PET imaging of brain tumors: Comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006. 47: 904-11
6. Cherian PJ, Radhakrishnan VV, Radhakrishnan K. The significance of corpora amylacea in mesial temporal lobe epilepsy. Neurol India. 2003. 51: 277-9
7. Chung MH, Horoupian DS. Corpora amylacea: A marker for mesial temporal sclerosis. J Neuropathol Exp Neurol. 1996. 55: 403-8
8. Das A, Balan S, Mathew A, Radhakrishnan V, Banerjee M, Radhakrishnan K. Corpora amylacea deposition in the hippocampus of patients with mesial temporal lobe epilepsy: A new role for an old gene?. Indian J Hum Genet. 2011. 17: S41-7
9. Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol. 1999. 20: 1252-7
10. Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996. 14: 1113-6
11. Gray F, Gherardi R, Marshall A, Janota I, Poirier J. Adult polyglucosan body disease (APBD). J Neuropathol Exp Neurol. 1988. 47: 459-74
12. Guzman-De-Villoria JA, Mateos-Perez JM, Fernandez-Garcia P, Castro E, Desco M. Added value of advanced over conventional magnetic resonance imaging in grading gliomas and other primary brain tumors. Cancer Imaging. 2014. 14: 35-
13. Kawamura T, Morioka T, Nishio S, Fukui K, Fukui M. Temporal lobe epilepsy and corpora amylacea in the hippocampus: Xlinicopathologic correlation. Neurol Res. 2002. 24: 563-9
14. Kishnani PS, Chuang TP, Bali D, Koeberl D, Austin S, Weinstein DA. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet. 2009. 18: 4781-90
15. Lossos A, Barash V, Soffer D, Argov Z, Gomori M, Ben-Nariah Z. Hereditary branching enzyme dysfunction in adult polyglucosan body disease: A possible metabolic cause in two patients. Ann Neurol. 1991. 30: 655-62
16. Lossos A, Meiner Z, Barash V, Soffer D, Schlesinger I, Abramsky O. Adult polyglucosan body disease in Ashkenazi Jewish patients carrying the Tyr329Ser mutation in the glycogen-branching enzyme gene. Ann Neurol. 1998. 44: 867-72
17. Mochel F, Schiffmann R, Steenweg ME, Akman HO, Wallace M, Sedel F. Adult polyglucosan body disease: Natural History and Key Magnetic Resonance Imaging Findings. Ann Neurol. 2012. 72: 433-41
18. Noguchi K, Watanabe N, Nagayoshi T, Kanazawa T, Toyoshima S, Shimizu M. Role of diffusion-weighted echo-planar MRI in distinguishing between brain brain abscess and tumour: A preliminary report. Neuroradiology. 1999. 41: 171-4
19. Radhakrishnan A, Radhakrishnan K, Radhakrishnan VV, Mary PR, Kesavadas C, Alexander A. Corpora amylacea in mesial temporal lobe epilepsy: Xlinico-pathological correlations. Epilepsy Res. 2007. 74: 81-90
20. Robitaille Y, Carpenter S, Karpati G, DiMauro SD. A distinct form of adult polyglucosan body disease with massive involvement of central and peripheral neuronal processes and astrocytes: A report of four cases and a review of the occurrence of polyglucosan bodies in other conditions such as Lafora's disease and normal ageing. Brain. 1980. 103: 315-36
21. Savage G RF, Halmagy M, Blazely A, Harper C. Stable neuropsychological defitis in adult polyglucosan disease. J Clin Neurosci. 2007. 14: 473-7
22. Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000. 217: 331-45
23. Schroder JM, May R, Shin YS, Sigmund M, Nase-Huppmeier S. Juvenile hereditary polyglucosan body disease with complete branching enzyme deficiency (type IV glycogenosis). Acta Neuropathol. 1993. 85: 419-30
24. Tung GA, Evangelista P, Rogg JM, Duncan JA. Diffusion-weighted MR imaging of rim-enhancing brain masses: Is markedly decreased water diffusion specific for brain abscess?. AJR Am J Roentgenol. 2001. 177: 709-12
25. Tung GA, Rogg JM. Diffusion-weighted imaging of cerebritis. AJNR Am J Neuroradiol. 2003. 24: 1110-3
26. Ubogu EE, Hong ST, Akman HO, Dimauro S, Katirji B, Preston DC. Adult polyglucosan body disease: A case report of a manifesting heterozygote. Muscle Nerve. 2005. 32: 675-81
27. Ziemssen F, Sindern E, Schroder JM, Shin YS, Zange J, Kilimann MW. Novel missense mutations in the glycogen-branching enzyme gene in adult polyglucosan body disease. Ann Neurol. 2000. 47: 536-40