- Department of Neurosurgery, Sher I Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
Correspondence Address:
Arif H. Sarmast
Department of Neurosurgery, Sher I Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
DOI:10.4103/sni.sni_45_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Syed M. Andrabi, Arif H. Sarmast, Altaf R. Kirmani, Abdul R. Bhat. Cranioplasty: Indications, procedures, and outcome – An institutional experience. 26-May-2017;8:91
How to cite this URL: Syed M. Andrabi, Arif H. Sarmast, Altaf R. Kirmani, Abdul R. Bhat. Cranioplasty: Indications, procedures, and outcome – An institutional experience. 26-May-2017;8:91. Available from: http://surgicalneurologyint.com/surgicalint-articles/cranioplasty-indications-procedures-and-outcome-an-institutional-experience/
Abstract
Background:Cranioplasty, the repair of a skull vault defect by insertion of an object (bone or nonbiological materials such as metal or plastic plates), is a well-known procedure in modern neurosurgery. Brain protection and cosmetic aspects are the major indications of cranioplasty. A retroprospective study was conducted for evaluating the indications, materials used, complications, and outcome of cranioplasty.
Methods:This study was prospective from August 2013 to September 2015 and retrospective from August 2010 to July 2013. In the retrospective study, patients files were retrieved from the mentioned date (August 2010 to July 2013) from the medical records and the findings were recorded. Abstracted data included age at the time of cranioplasty (years), sex (male or female), medical comorbidities (hypertension, diabetes), indications for craniectomy [Road traffic accident (RTA), fall from height (FFH), hit by stone or cricket ball, physical assault, stroke, infection, shell injury, bullet injury, and intraoperative swelling], laterality of cranioplasty (bilateral, unilateral, or bifrontal), time between craniectomy and cranioplasty (weeks), type of graft (autologous or artificial), type of prosthesis if used (methylmethacrylate, titanium), storage of bone flap if used (subcutaneous or deep freezer), operative time (minutes), and complications fallowing cranioplasty.
Results:Of the 236 patients included in the study, maximum were in the age group of 21–30 years i.e., 30.93% (n = 73). Mean age of the patients was 33.44 years. A total of 196 (83.05%) were autologous and 40 (16.95%) were artificial. Out of the 40 patients who underwent artificial cranioplasty, 36 (15.25%) had methylmethacrylate graft and 4 (1.7%) had titanium mesh implant. Bone was not preserved in 16.95% (n = 40), preserved in subcutaneous tissue in abdominal wall in 2.54% (n = 6), and preserved in deep freezer in 80.51% (n = 190) of the patients.
Conclusion:Cranioplasty as a procedure is not without complications; however, if performed properly and at proper time with an aseptic technique, good results are achieved
Keywords: Artificial, autologous, cranioplasty, titanium mesh
INTRODUCTION
Cranioplasty, the repair of a skull vault defect by insertion of an object (bone or nonbiological materials such as metal or plastic plates), is a well-known procedure in modern neurosurgery. Brain protection and cosmetic aspects are the major indications of cranioplasty.[
Cranioplasty can avoid the recurrence of brain damage, can achieve the plastic effect, can protect the patient from cerebral seizures, can relieve the syndrome of trephine (i.e., headaches, dizziness, intolerance of vibration and noise, irritability, fatigability, loss of motivation and concentration, depression, and anxiety),[
MATERIALS AND METHODS
The Department of Neurosurgery, Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Kashmir has been performing the procedure of cranioplasty since 1982. This study was prospective from August 2013 to September 2015 and retrospective from August 2010 to July 2013. In the prospective study, patients who presented to the Department of Neurosurgery SKIMS with a craniotomy defect and underwent cranioplasty from August 2013 to September 2015 were included in the study. In retrospective study, the files of the patients were retrieved from the mentioned date (August 2010 to July 2013) from the medical records and the findings were recorded. Abstracted data included age at the time of cranioplasty (years), sex (male or female), medical comorbidities (hypertension, diabetes), indications for craniectomy [road traffic accidents (RTA), fall from height (FFH), hit by stone or cricket ball, physical assault, stroke, infection, shell injury, bullet injury, and intraoperative swelling], laterality of cranioplasty (bilateral, unilateral, or bifrontal), time between craniectomy and cranioplasty (weeks), type of graft (autologous or artificial), type of prosthesis if used (methylmethacrylate, titanium), storage of bone flap if used (subcutaneous or deep freezer), operative time (minutes), and complications fallowing cranioplasty. We included all infections, wound breakdowns, cases of significant bone resorption, and symptomatic hematoma requiring reoperation. The indications for reoperations were recorded separately. Patients were prospectively followed up through outpatient department (OPD) and by phone till March 2016. A proforma for the symptoms, signs, procedure, and outcome for each patient was used to record the data. The data was compiled and computed for various results. A total of 236 patients were included in the study.
Statistical analysis
Data was described as mean ± SD (Standard deviation) and percentages. Chi-square test, Fisher's exact test, and independent t-test were used for data analysis.
RESULTS
A total of 236 patients were included in the study.
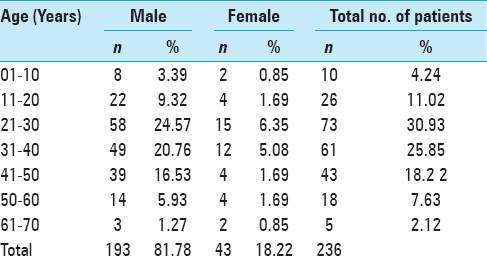
Age and gender distribution of the studied patients
Age and gender distribution of the studied patients is shown in
Type of the graft used
Of the 236 procedures, 196 (83.05%) were autologous and 40 (16.95%) were artificial. Out of the 40 patients who underwent artificial cranioplasty, 36 (15.25%) had methylmethacrylate graft and 4 (1.7%) had titanium mesh implant.
Type of the preservation method
Bone was not preserved in 16.95% (n = 40), preserved in subcutaneous tissue in abdominal wall in 2.54% (n = 6), and preserved in deep freezer in 80.51% (n = 190) of the patients.
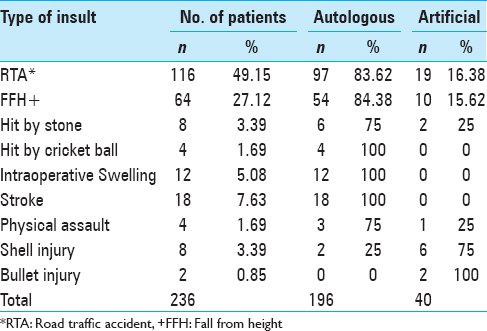
Reason for removal of bone flaps
The initial diagnosis of the patients included RTA, FFH, hit by stone, hit by cricket ball, intraoperative swelling, stroke, physical assault, shell injury, and bullet injury. The most common cause of the bone flap removal was RTA (49.15%, n = 116) followed by FFH (27.12%, n = 64), and stroke (7.63%, n = 18), respectively, as depicted in
Laterality of cranioplasty
Regarding laterality of the defect, the most common cranial defect was unilateral (94.92%, n = 224) followed by bilateral (4.24%, n = 10), and bifrontal (0.84%, n = 2).
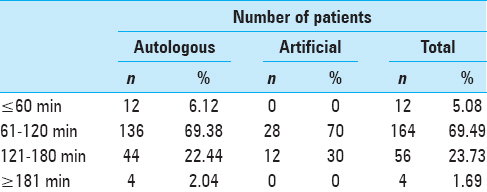
Time of the surgical procedure
With respect to the time of surgical procedure, most patients were operated between 61–120 minutes (69.49%, n = 164) followed by between 121–180 minutes 23.73% (n = 56), with a mean operative time of 119.51 minutes. The mean operative time of autologous and artificial cranioplasty was 118.34 ± 34.58 minutes and 125.25 ± 27.07 minutes, respectively (P = 0.235), as depicted in
Complication following cranioplasty
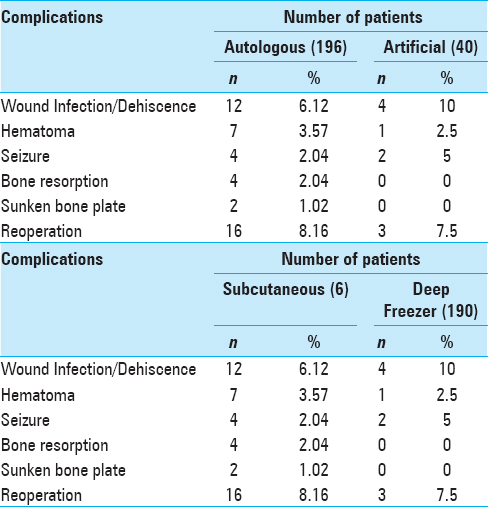
Complications were noted in 15.25% (n = 36) of the patients; wound infection/dehiscence 6.78% (n = 16) was the most common complication encountered. Postoperative hematoma was also a significant complication fallowing cranioplasty. Other complications included seizures 2.54% (n = 6), bone resorption 1.69% (n = 4), and sunken bone plate 0.85% (n = 2). Nineteen out of the 36 patients having complications had to undergo reoperation. Complications were more common in males 16.06% (31 out of 193 males) than females 11.63% (2 out of 43 females).
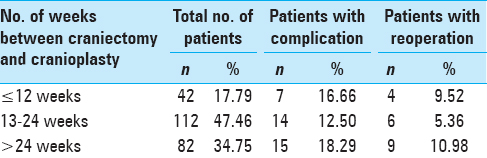
Time between craniectomy and cranioplasty and ensuing complications
Most of the patients 47.46% (n = 11) were operated between 13 and 24 weeks after the primary procedure. Complications were most commonly seen in patients (18.29%, n = 15) who had undergone cranioplasty after 6 months of the initial primary procedure (P = 0.520). Reoperation rate of 10.98% was seen in patients undergoing cranioplasty greater than 24 weeks from the primary procedure (P = 0.316), as depicted in
Type of the graft used and ensuing complications
Wound infection was seen in 10% (n = 4) of the patients who had undergone artificial cranioplasty compared to 6.12% (n = 12) of the patients who had undergone autologous cranioplasty. Net complication rate of 14.79% was seen in autologous group compared to 17.5% in the artificial group (P = 0.665) [
Type of the autologous bone storage and ensuing complications
The most common method of bone storage was deep freezer 80.51% (n = 190). Complications as well as reoperation rate was most commonly seen in subcutaneous bone storage [
Complications recurring reoperation
Reoperation rate was most commonly seen in patients who had undergone bilateral cranioplasty 20% (n = 2) compared to patients who had undergone unilateral cranioplasty 7.59% (n = 17). Reoperation rate was slightly higher in patients who had undergone autologous cranioplasty.
Complications with respect to type of injury
Complications were noted in 15.25% (n = 36) of the patients. Out of the 36 patients, 63.89% (n = 23) were having open type of injury whereas 36.11% (n = 13) where having closed type of injury.
DISCUSSION
It has been documented that cranioplasties were performed by the Incas many centuries ago.[
A total of 236 patients who were admitted in the Department of Neurosurgery of SKIMS, Soura, Srinagar and had undergone cranioplasty from August 2010 to September 2015 were included in the study.
Of the 236 patients included in the study maximum were in the age group of 21–30 years, i.e., 30.93% (n = 73). Mean age of the patients was 33.44 years. Among all the patients, 81.78% (n = 193) were males and 18.22% (n = 43) were females. Mean age of males was 33.4 years and of females were 33.58. Hamandi et al. reported in their study that 85.7% (n = 12) were males and 14.3% were females, and maximum were in the age group of 21–30 years, which is somewhat in accordance to our study.[
Regarding preservation method, bone was not preserved in 16.95% (n = 40), preserved in subcutaneous tissue in abdominal wall in 2.54% (n = 6), and preserved in deep freezer in 80.51% (n = 190) of the patients. Most surgeons prefer subcutaneous pocket because majority are of the opinion that keeping bone in the subcutaneous pocket will ensure viability of bone, resulting in better fusion and less infection rate. However, this adds to the morbidity of the procedure by prolonging the operation time and blood loss, which is very important factor in prognosis especially during decompressive craniectomy. Moreover, patient discomfort and wound complications including infection, hematoma, and seroma are important factors discouraging keeping bone in subcutaneous pocket. Lal et al. in their study concluded that the current literature suggests that the storage of bone flaps in freezers is the most common method, which is somewhat in accordance to our study.[
The initial diagnosis of the patients included RTA, FFH, hit by stone, hit by cricket ball, intraoperative swelling, stroke, physical assault, shell injury, and bullet injury. The most common cause of the bone flap removal was RTA (49.15%, n = 116) followed by FFH (27.12%, n = 64), and stroke (7.63%, n = 18). Lal et al. in their study reported that the leading primary pathology was traumatic brain injuries including both blunt as well as penetrating injuries, which is somewhat in accordance to our study.[
Regarding laterality of the defect, the most common cranial defect was unilateral (94.92%, n = 224) followed by bilateral (4.24%, n = 10), and bifrontal (0.84%, n = 2). Various studies on cranioplasty have shown that unilateral defect is the most common cranial defect. Basheer et al. in their study of 114 patients reported that 90.35% (n = 103) were unilateral, 5.26% (n = 6) were bilateral, and 4.39% (n = 5) were bifrontal, which is somewhat in accordance to our study.[
With respect to time of surgical procedure most of the patients were operated within 61–120 minutes (69.49%, n = 164) followed by within 121–180 minutes 23.73% (n = 56), with a mean operative time of 119.51 minutes. The mean operative time of autologous and artificial cranioplasty was 118.34 ± 34.58 minutes and 125.25 ± 27.07 minutes, respectively, with P value of 0.235, which is considered not significant. Al-Shalchy conducted a study in which 90% (n = 18) of the patients were operated within 1–3 hours, which is somewhat in accordance to our study.[
Complications were noted in 15.25% (n = 36) of the patients and wound infection/dehiscence 6.78% (n = 16) was the most common complication encountered. Postoperative hematoma was also a significant complication fallowing cranioplasty. The other complications included seizures 2.54% (n = 6), bone resorption 1.69% (n = 4), and sunken bone plate 0.85% (n = 2). Nineteen out of the 36 patients having complications had to undergo reoperation. Complications were more common in males 16.06% (31 out of 193 males) than females 11.63% (2 out of 43 females). Walcott et al. in their study reported that wound infection 12.13% (n = 29) was the most common complication fallowing cranioplasty. They had a net complication rate of 23.85% (n = 57), which is somewhat in accordance to our study.[
Most of the patients 47.46% (n = 11) were operated within 13–24 weeks after the primary procedure. Complications were most commonly seen in patients (18.29%, n = 15) who had undergone cranioplasty after 6 months of the initial primary procedure. The reasons for delayed cranioplasty include patients deemed medically or neurologically unstable until the point of intervention or nonresolution of cerebral edema or centralized nature of neurosurgical care at our place where there are logistic difficulties in operating patients early. The P value with respect to time between craniotomy and cranioplasty and the ensuing complications was 0.520, which is not significant. Reoperation rate of 10.98% was seen in patients undergoing cranioplasty greater than 24 weeks from the primary procedure with a P value of 0.316, which is considered not significant. The optimal timing of cranioplasty following craniectomy is intensely debated. Studies have been performed that either support or refute its influence on postcranioplasty infection.[
Complication was seen in 14.79% (n = 29) of the patients who had undergone autologous cranioplasty compared to 17.5% (n = 7) of the patients who had undergone artificial cranioplasty. Net complication rate of 14.79% was seen in the autologous group compared to 17.5% in the artificial group, with a P value of 0.665, which is considered not significant. Basheer et al. in their study reported that the complication rate was slightly higher in the artificial group.[
The most common method of bone storage was deep freezer 80.51% (n = 190). Complications as well as reoperation rate was most commonly seen in subcutaneous bone storage. Basheer et al. reported complication rate of 21.4% (n = 8), with a reoperation rate of 14.3% (n = 12) seen in subcutaneous bone storage and complication rate of 22.22% (n = 4) with a reoperation rate of 11.1% (n = 2) seen in patients whose bone was stored in deep freezer.[
Reoperation rate was most commonly seen in patients who had undergone bilateral cranioplasty 20% (n = 2) compared to patients who had undergone unilateral cranioplasty 7.59% (n = 17). Reoperation rate was also higher in patients who had undergone autologous cranioplasty. Basheer et al. reported reoperation rate of 13.5% (n = 14) seen in patients who had undergone unilateral cranioplasty compared to 16.7% (n = 1) in patients who had undergone bilateral cranioplasty. Reoperation rate of 13.3% (n = 14) was seen in the autologous group compared to 16.7% noted in the artificial group.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agner C, Dujovny M, Gaviria M. Neurocognitive assessment before and after cranioplasty. Acta Neurochir. 2002. 144: 1033-40
2. Al-Shalchy AK. Cranioplasty the use synthetic (Acrylic) or Autograft. J Fac Med Baghdad. 2010. 52: 30-1
3. Basheer N, Gupta D, Mahapatra AK, Gurjar H. Cranioplasty following decompressive craniectomy in traumatic brain injury: Experience at level-I apex trauma centre. Indian J Neurotrauma. 2010. 7: 139-44
4. Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J. Cranioplasty after post injury decompressive craniectomy: Is timing of the essence?. J Trauma. 2010. 69: 270-4
5. Bhat AR, Kirmani AR, Nizami F, Kumar A, Wani MA. Sunken brain and scalp flap” syndrome following decompressive “extra-craniectomy. Indian J Neurotrauma. 2011. 8: 105-8
6. Carvi Y, Nievas MN, Höllerhage HG. Early combined cranioplasty and programmable shunt in patients with skull bone defects and CSF-circulation disorders. Neurol Res. 2006. 28: 139-44
7. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. Clinical article. J Neurosurg. 2010. 112: 1120-4
8. Chun HJ, Yi HJ. Efficacy and safety of early cranioplasty, at least within 1 month. J Craniofac Surg. 2011. 22: 203-7
9. Donald JP. Cranial defect & cranioplasty. Clin Neurosurg. 1996. 275: 2783-95
10. Dujovny M, Agner C, Aviles A. Syndrome of the trephined: Theory and facts. Crit Rev Neurosurg. 1999. 9: 271-8
11. Erdogan E, Düz B, Kocaoglu M, Izci Y, Sirin S, Timurkaynak E. The effect of cranioplasty on cerebral hemodynamics: Evaluation with transcranial Doppler sonography. Neurol India. 2003. 51: 479-81
12. Fodstad H, Ekstedt J, Friden H. CSF hydrodynamic studies before and after cranioplasty. Acta Neurochir Suppl. 1979. 28: 514-8
13. Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir. 1984. 70: 21-30
14. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
15. Hamandi YMH, Al-Khafaji AJ, Nema IS. Cranioplasty (Monomeric Acrylic Designed in Dental Laboratory Versus Methylmethacrylate Codman's Type). Postgrad Med J. 2011. 10: 198-203
16. Lal PK, Shamim MS. The evolution of cranioplasty: A review of graft types, storage options and operative technique. Pakistan J Neurol Sci. 2012. 7: 21-7
17. Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg. 2007. 18: 526-32
18. Moin H, Mohagheghzadeh P, Darbansheikh A. The use of frozen autogenous bone flap for cranioplasty. JRMS. 2005. 10: 395-7
19. Rifkinson-Mann S. Cranial surgery in ancient Peru. Neurosurgery. 1988. 23: 411-6
20. Rish BL, Dillon JD, Meirowsky AM, Caveness WF, Mohr JP, Kistler JP. Cranioplasty: A review of 1030 cases of penetrating head injury. Neurosurgery. 1979. 4: 381-5
21. Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997. 40: 588-603
22. Schaller B, Graf R, Sanada Y, Rosner G, Wienhard K, Heiss WD. Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res. 2003. 982: 31-7
23. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Wael F. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62
24. Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. The influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. Neurosurg Focus. 2000. 8: E9-
25. Won YD, Yoo DS, Kim KT, Kang SG, Lee SB, Kim DS. Cranioplasty effect on the cerebral hemodynamics and cardiac function. Acta Neurochir Suppl. 2008. 102: 15-20
26. Yang XJ, Hong GL, Su SB, Yang SY. Complications induced by decompressive craniectomies after traumatic brain injury. Chin J Traumatol. 2003. 6: 99-103
27. Zhang GL, Yang WZ, Jiang YW, Zeng T. Extensive duraplasty with autologous graft in decompressive craniectomy and subsequent early cranioplasty for severe head trauma. Chin J Traumatol. 2010. 13: 259-64