- Department of Neurosurgery, Swiss Hospital, Monterrey, Mexico.
- Department of Neurosurgery, Northeast National Medical Center, Monterrey, Mexico.
Correspondence Address:
J Javier Cuellar-Hernandez
Department of Neurosurgery, Northeast National Medical Center, Monterrey, Mexico.
DOI:10.25259/SNI_65_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: J Javier Cuellar-Hernandez1, Carlos Seañez2, Ramon Olivas-Campos2, Rodrigo Chavez2, Paulo M. Tabera-Tarello2, B. Manuel Serna-Roman2. Cryptococcal meningitis presenting as anterior spinal cord syndrome with accessory nerve palsy in immunocompetent patient: A case report. 19-Apr-2021;12:167
How to cite this URL: J Javier Cuellar-Hernandez1, Carlos Seañez2, Ramon Olivas-Campos2, Rodrigo Chavez2, Paulo M. Tabera-Tarello2, B. Manuel Serna-Roman2. Cryptococcal meningitis presenting as anterior spinal cord syndrome with accessory nerve palsy in immunocompetent patient: A case report. 19-Apr-2021;12:167. Available from: https://surgicalneurologyint.com/surgicalint-articles/10740/
Abstract
Background: Cryptococcus has a tropism for the nervous system with a higher prevalence of infection in immunosuppressed patients; it remains a major cause of human immunodeficiency virus (HIV)-related mortality worldwide. Neurological compromise caused by this microorganism mainly debuts as a meningeal syndrome, spinal involvement has been reported in literature, neuropathological assessments have found Cryptococci in spinal roots and meninges, with perineuritic adhesions probably explaining compromise lower cranial nerves and even spinal nerve roots.
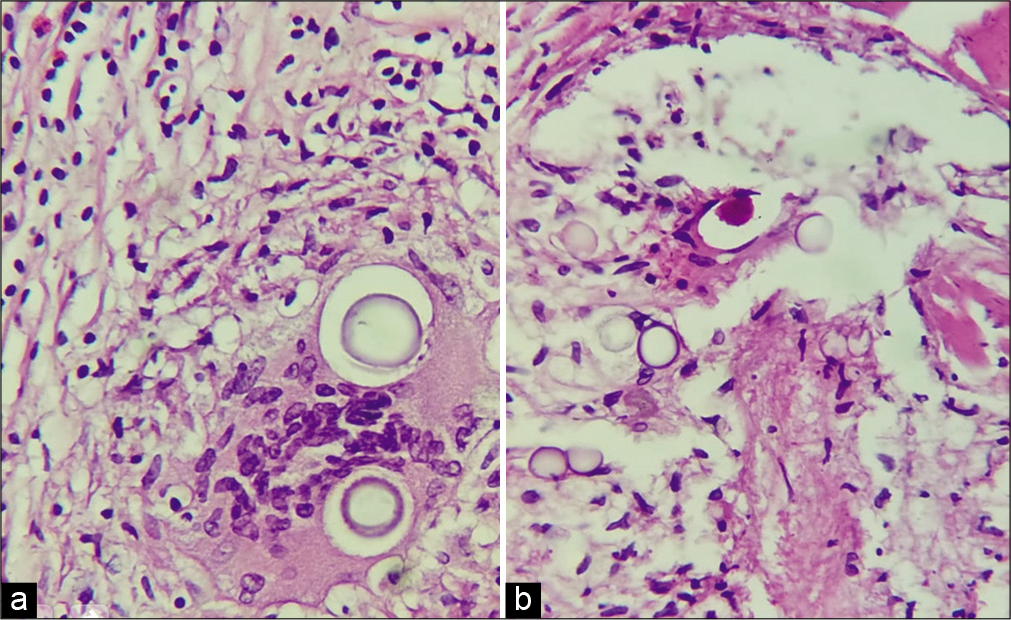
Case Description: 39-year-old male seronegative for HIV, with a surgical history of hydrocephalus treated with ventriculoperitoneal shut 1 year before, he presented with progressive weakness in the four extremities evolving to be disabling with bilateral accessory nerve palsy and loss of sensation below his neck. The MR imaging showed diffuse leptomeningeal thickening both supra and infratentorial and over the spinal canal up to C5 with a cystic formation shown in the craniocervical union causing compression of the medullary bulb. The patient underwent a medial suboccipital craniectomy with resection of the posterior arch of c1 for sampling and decompression, pathologically appears numerous spherical organisms that have a thick clear capsule and are surrounded by histiocytes forming a granuloma compatible with Cryptococcus. Postoperatively, the patient’s prior neurological deficits resolved.
Conclusion: It is an infrequently suspected pathology in immunocompetent patients, usually requiring only antifungal treatment with adjustment of immunosuppressive or antiretroviral management. In special and rare situations like our case as presenting with lower cranial nerve and spinal involvement, surgical treatment is a priority for the resolution of the pathology and improves disabling neurological deficit.
Keywords: Anterior spinalcord syndrome, Cryptococcus, Meningitis, Suboccipital craniectomy
INTRODUCTION
Cryptococcus has a tropism for the nervous system with a higher prevalence of infection in immunosuppressed patients; it remains a major cause of human immunodeficiency virus (HIV)-related mortality worldwide. Is an opportunistic infection that causes more than 100,000 HIV-related deaths each year.[
Neurological compromise caused by this microorganism mainly debuts as a meningeal syndrome characterized by headache, altered mental status, and other signs and symptoms include lethargy along with fever, stiff neck (both associated with an aggressive inflammatory response), nausea, and vomiting. Some patients who are HIV positive may have minimal or nonspecific symptoms at presentation. The duration of symptoms from onset to a presentation is usually 1 to 2 weeks in HIV cases and 6 to 12 weeks in nonHIV cases.[
Cryptococcal meningitis with spinal involvement has been reported in literature, neuropathological assessments have found Cryptococci in spinal roots and meninges, with perineuritic adhesions[
CASE PRESENTATION
A 39-year-old male, inhabitant of Mexico northeastern endemic area for Cryptococcus with a surgical history of hydrocephalus treated with ventriculoperitoneal shut 1 year before, he presented 3 months of progressive weakness in the four extremities evolving to be disabling with bilateral accessory nerve palsy and loss of sensation below his neck.
On physical examination, he had increased deep tendon reflexes and 2/5 muscle strength in his bilateral upper and lower extremities with a sensory level of C3, intact proprioception and vibration, with absent pain and temperature sensation.
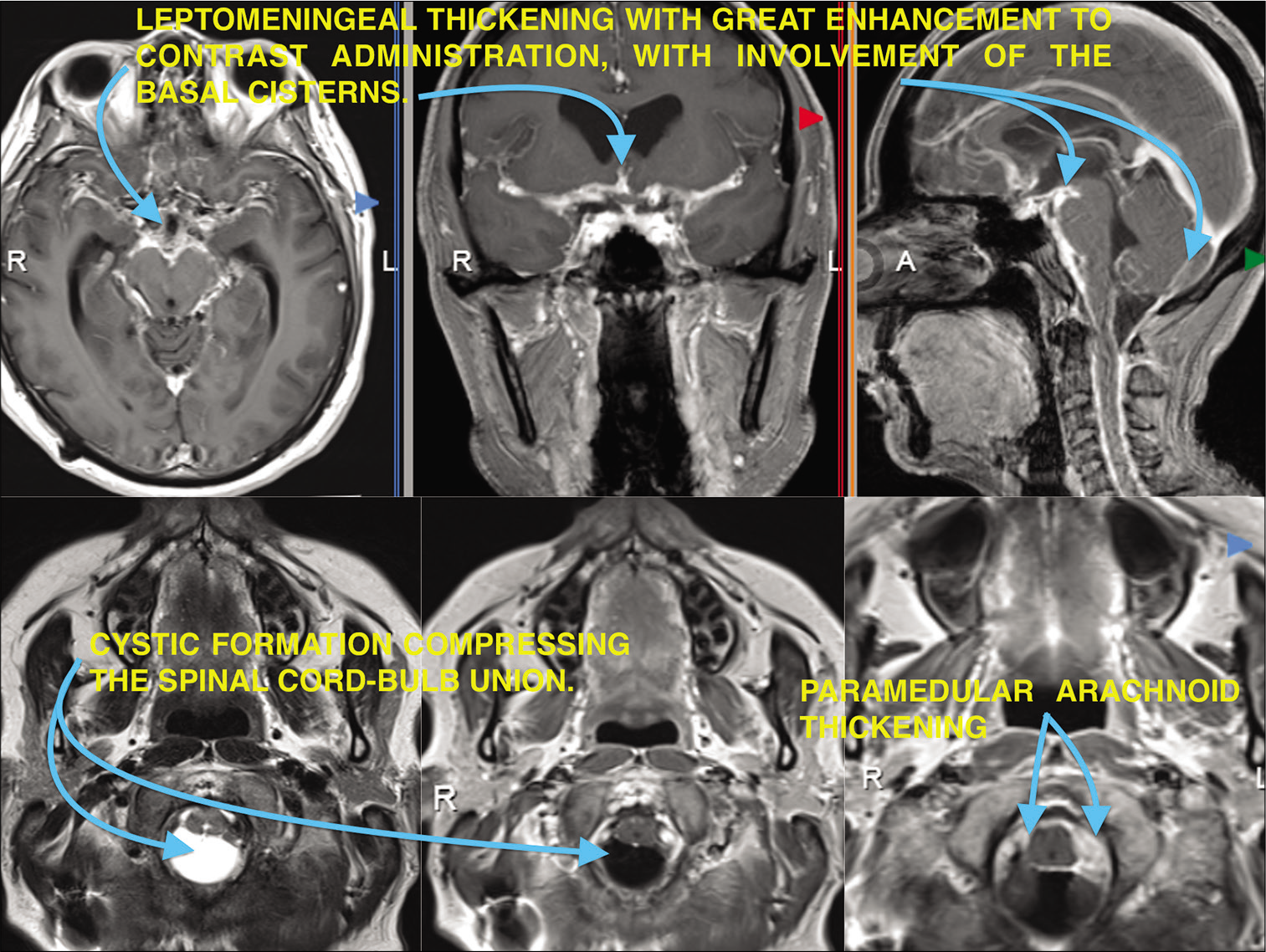
The magnetic resonance imaging of the brain and craniocervical junction with and without contrast showed diffuse leptomeningeal thickening both supra and infratentorial and over the spinal canal up to C5, with great enhancement to contrast administration, with involvement in ambiens, interpeduncular, prepontine, sylvian, premedullary, and Magna cisterns. Cystic formation is also shown in the cranio-cervical union causing compression of the medullary bulb [
The patient was seronegative for HIV.
Surgery
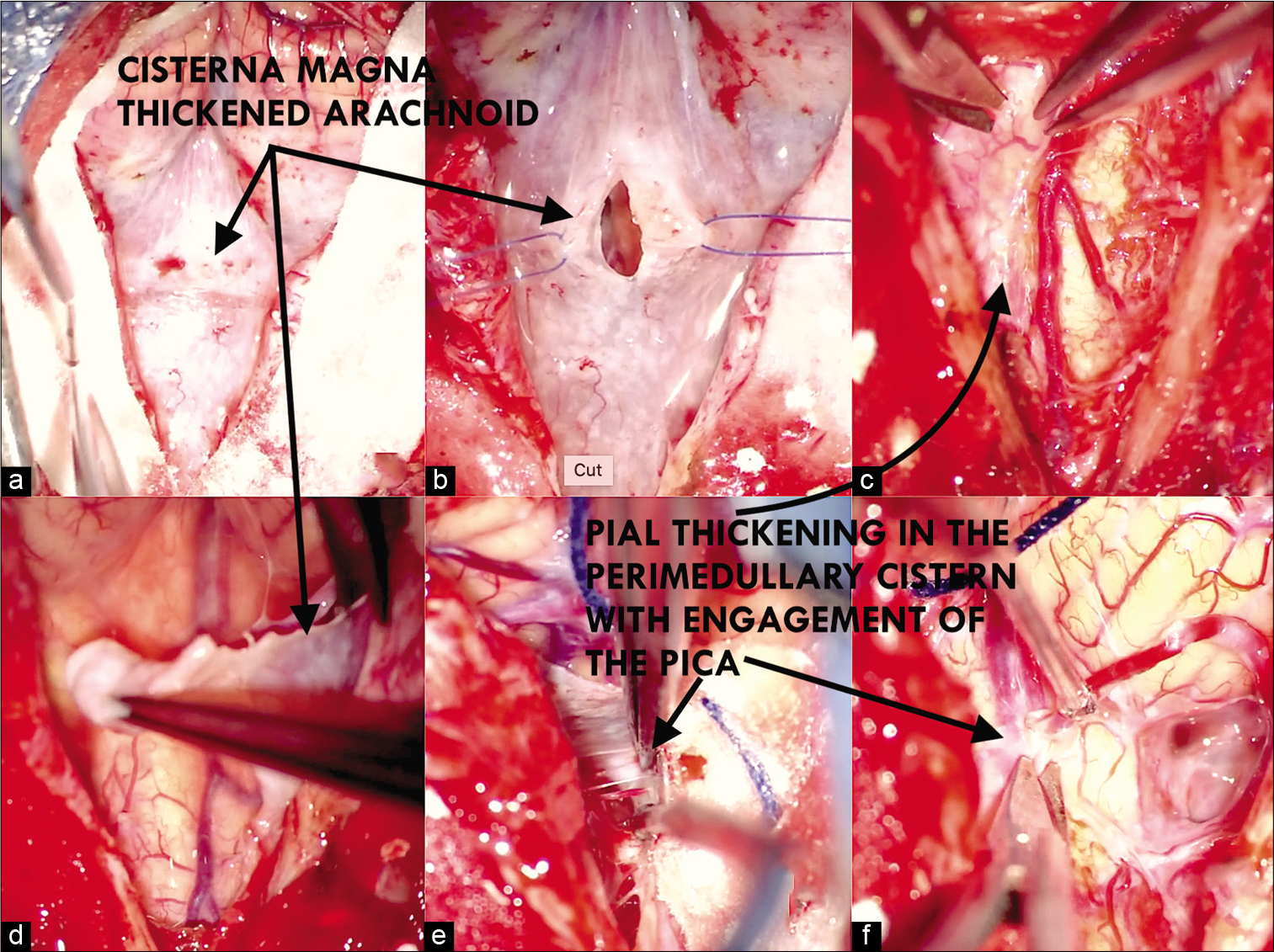
The patient underwent a medial suboccipital craniectomy with resection of the posterior arch of c1, intradurally found meningeal layer of the dura with multiple adhesions to the thickened arachnoid with multiple nodules on its surface with poor vascularization, opening of the arcanoid with partial resection on the cistern magna and on the bulbomedullary cistern with sampling for definitive pathological study, a sample of the pia mater was taken, which was thickened with the same characteristics of the arachnoid. Pathological cerebrospinal fluid (CSF) found in an arachnoid cyst formed by multiple adhesions, cerebellar tonsils were released with opening of the medullary velum until the IV ventricle was identified, choroid plexuses of calcified Lushka foramens with whitish granulations inside. Cerebellar hemisphere, biventral lobe as well as superior and inferior semilunar lobes with pial narrowing maintaining arterial integrity. Firm adhesions are observed on emergence of both accessory nerves as well as bilateral IX, X, XI, XII, and PICA [
Figure 2:
Intraoperative view, multiple adhesions to the thickened arachnoid with multiple nodules on its surface (a and b), the pia mater was thickened with the same characteristics of the arachnoid (c and d). Cerebellar tonsils were released with opening of the medullary velum until the IV ventricle was identified, Firm adhesions are observed on emergence of both accessory nerves as well as bilateral IX, X, XI, XII, and PICA (e and f).
DISCUSSION
Cryptococcosis is an infectious disease that frequently affects the lungs and central nervous system. It is caused by Cryptococcus neoformans and Cryptococcus Gattii, the first is associated with immunocompromised patients due to organ transplantation, especially in patients with advanced HIV disease and low CD4 cell count, and the second is more commonly associated with immunocompetent patients.[
In the central nervous system, it causes encephalitis and meningitis, affecting different levels of the central nervous system, spinal arachnoiditis occurs more sporadically.[
The clinical and radiological conditions of this patient led us to consider arachnoid cysts and epidermoid cysts, including tuberculosis arachnoiditis. Offer early treatment avoids possible complications such as the development of cerebral infarcts and intracranial hypertension, which usually require CSF shunt systems in about 50% of cases.[
First-line of treatment is medical with antifungal drugs, with in consensus with international guidelines, amphotericin followed by treatment with azoles for 12 months or more, presents a partial or total recovery of lost functions.[
CONCLUSION
It is an infrequently suspected pathology in immunocompetent patients, usually requiring only antifungal treatment with adjustment of immunosuppressive or antiretroviral management. In special situations like our case as presenting with lower cranial nerve and spinal involvement, surgical treatment is a priority for the resolution of the pathology and improves disabling neurological deficit. The participation of the infectologist is essential in the establishment and monitoring of antifungal treatment.
Dearation of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adsul N, Kalra KL, Jain N, Haritwal M, Chahal RS, Acharya S. Thoracic cryptococcal osteomyelitis mimicking tuberculosis: A case report. Surg Neurol Int. 2019. 10: 81
2. Beardsley J, Sorrell TC, Chen SC. Central nervous system cryptococcal infections in non-HIV infected patients. J Fungi (Basel). 2019. 5: 71
3. Calvo A, Hernández P, Spagnuolo E, Johnston E. Surgical treatment of intracranial hypertension in encephalic cryptococcosis. Br J Neurosurg. 2003. 17: 450-5
4. Cherian J, Atmar RL, Gopinath SP. Shunting in cryptococcal meningitis. J Neurosurg. 2016. 125: 177-86
5. Kanaly CW, Selznick LA, Cummings TJ, Adamson DC. Cerebellar cryptococcoma in a patient with undiagnosed sarcoidosis: Case report. Neurosurgery. 2007. 60: E571
6. May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol. 2016. 14: 106-17
7. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016. 30: 179-206
8. Panackal AA, Komori M, Kosa P, Khan O, Hammoud DA, Rosen LB. Spinal arachnoiditis as a complication of cryptococcal meningoencephalitis in non-HIV previously healthy adults. Clin Infect Dis Adv Access. 2016. 183: 1-27
9. Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc. 2013. 124: 61-79
10. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010. 50: 291-322
11. Santander XA, Gutiérrez-González R, Cotúa C, Tejerina E, Rodríguez GB. Intraventricular cryptococcoma mimicking a neoplastic lesion in an immunocompetent patient with hydrocephalus: A case report. Surg Neurol Int. 2019. 10: 115
12. Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A. Cryptococcal meningitis: Epidemiology, immunology, diagnosis and therapy. Nat Rev Neurol. 2017. 13: 13-24