- Department of Neurosurgery, Detroit Medical Center, Wayne State University, Detroit, Michigan,
- Department of Surgery, Swedish Neuroscience Institute, Seattle, WA, United States.
Correspondence Address:
Dia Radi Halalmeh
Department of Neurosurgery, Detroit Medical Center, Wayne State University, Detroit, Michigan,
DOI:10.25259/SNI_568_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Manan Shah, Catherine Peterson, Emre Yilmaz, Dia Radi Halalmeh, Marc Moisi. Current advancements in the management of spinal cord injury: A comprehensive review of literature. 03-Jan-2020;11:2
How to cite this URL: Manan Shah, Catherine Peterson, Emre Yilmaz, Dia Radi Halalmeh, Marc Moisi. Current advancements in the management of spinal cord injury: A comprehensive review of literature. 03-Jan-2020;11:2. Available from: https://surgicalneurologyint.com/surgicalint-articles/9833/
Abstract
Background: Spinal cord injury (SCI) carries debilitating lifelong consequences and, therefore, requires careful review of different treatment strategies.
Methods: An extensive review of the English literature (PubMed 1990 and 2019) was performed regarding recent advances in the treatment of SCI; this included 46 articles written over 28 years.
Results: Results of this search were divided into five major modalities; neuroprotective and neuroregenerative pharmaceuticals, neuromodulation, stem cell-based therapies, and various external prosthetic devices. Lately, therapeutic strategies were mainly focused on two major areas: neuroregeneration and neuroprotection.
Conclusion: Despite recent advancements, more clinical trials on a larger scale and further research are needed to provide better treatment modalities of this devastating neurological disease.
Keywords: Exoskeleton, Neuromodulation, Spinal cord injury, Spine, Stem cells, Trauma
INTRODUCTION
Spinal cord injury (SCI) is a devastating illness resulting in neurological deficits and poor quality of life. It has an annual incidence of 15–40 cases per million and a prevalence of more than 1 million cases in North America.[
This literature review focuses on the advances in pharmacology, stem cell technologies, neuromodulation, and external prosthetics. Several pharmacological therapies have already been tested in the past and are currently being investigated. Further, both neuroprotective and neuroregenerative drugs are being implemented in clinical trials.[
MATERIALS AND METHODS
Peer-reviewed articles were searched through PubMed using search terms “acute SCI,” “SCI treatment,” “neuromodulation,” “stem cell therapy for SCI,” “SCI pharmaceuticals,” and “SCI exoskeleton from 1990 to 2019 (English journals). Using appropriate inclusion and exclusion criteria, 46 peer-reviewed articles were used. All studies focused on current advancements in the management of SCI, including stem cell therapies, neuromodulation, and external prosthetics.
RESULTS AND DISCUSSION
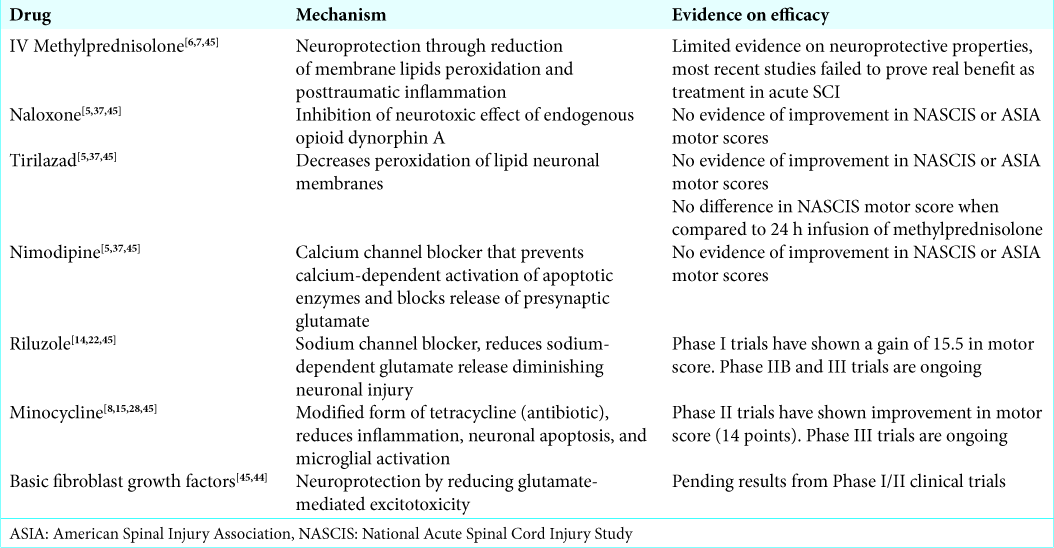
Neuroprotective and neuroregenerative pharmaceuticals [ Tables 1 and 2 ]
Methylprednisolone
Several neuroprotective and neuroregenerative pharmaceutical drugs have been investigated for SCI management. A well-known neuroprotective agent, methylprednisolone, has been associated with improved neurological outcomes. It decreases the peroxidation of membrane lipids and posttraumatic inflammation.[
Naloxone, tirilazad, and nimodipine
Three drugs, naloxone, tirilazad, and nimodipine, were studied for their neuroprotective abilities. They all have Phase III randomized controlled trials which have not shown any difference in NASCIS motor score recovery or the American Spinal Injury Association (ASIA) motor score between treatment and placebo groups.[
Riluzole
Riluzole, a sodium channel blocker approved for the treatment of amyotrophic lateral sclerosis, has been studied in preclinical models of SCI. It diminishes secondary injury by blocking activation of sodium channels and reducing release of neuronal glutamate.[
Minocycline
Minocycline, a modified form of tetracycline, is another neuroprotective agent that has shown some promise in animal models.[
Fibroblast growth factor
Basic fibroblast growth factor has shown to provide neuroprotection by improving functional and respiratory parameters in animal models by reducing glutamate-mediated excitotoxicity.[
GM-1 ganglioside (Sygen)
A neuroregenerative agent, GM-1 ganglioside (Sygen) has been shown to enhance axonal regeneration in laboratory studies.[
Cethrin
Cethrin is a permeable paste that can be applied to spinal cord dura postinjury that is a combination of a bacterial-derived toxin, BA-210, and a biohemostatic adhesive. It inhibits the Rho pathway of inhibitory proteins and promotes axonal growth in vitro.[
Anti-Nogo
Another neuroregenerative drug, anti-Nogo, is a monoclonal antibody made to bind to Nogo-A, and has been shown to promote neural regeneration.[
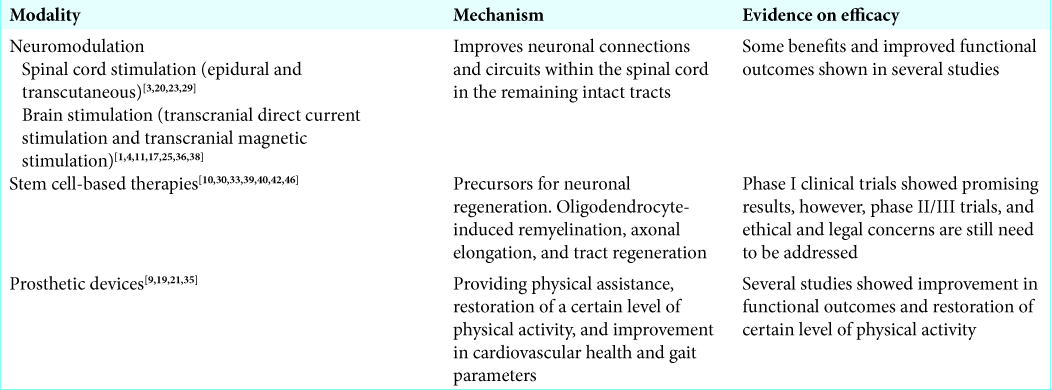
Neuromodulation [ Table 3 ]
It is well known that neuromodulation, the use of electrical stimulation to alter neuronal circuitry, has been tried in various neurological disorders including SCI. Neuroplasticity-mediated functional recruitment of axons (particularly spared axons) to potentiate sprouting, regeneration, and formation of new interconnections between neurons forms the basis of modern neuromodulation techniques. This is complemented with the presence of some intact ascending and descending circuits in patients with SCI, making neuromodulation a feasible option.[
Activity-dependent plasticity
Moreover, the concept of activity-dependent plasticity has been recently employed to achieve substantial improvements in motor function, based on the recent finding that neurorehabilitation is the only treatment option which can be offered to SCI patients for long-term improvement in motor function.[
Spinal cord stimulation
With respect to spinal cord stimulation, epidural spinal stimulation has well been tested in patients with chronic pain and most recently in patients with SCI. This method involves surgical placement of electrodes onto the dorsal surface of the spinal cord.[
Brain stimulation for SCI
Brain stimulation for SCI is also currently being employed. Transcranial direct current stimulation and transcranial magnetic stimulation are two main approaches that are being used to augment the neuronal plasticity between the spinal cord and the brain in individuals with SCI.[
Brain–machine interfaces
Brain–machine interfaces are another modern tool for patients with SCI. These devices, which can be used to control various prosthetic devices such as the exoskeleton as well as directly stimulate paralyzed muscles, have already demonstrated improved outcomes in patients with SCI through several recent studies.[
Stem cell-based therapies [ Table 3 ]
Stem cell-based therapies and cellular scaffolds have yielded promising progress with respect to neuronal repair.[
In vitro manipulation of the embryonic stem cells (ESCs)
Recently, in vitro manipulation of the ESCs differentiation to neuronal and glial lineages under controlled conditions has shown promising results after transplantation in animal models of acute SCI.[
Various cell-based therapies
Despite extensive research exploring various cell-based therapies such as transplantation of oligodendrocyte precursors, induced pluripotent stem cells, bone marrow-derived (BM-MSCs), adipose-derived (AD-MSCs), and umbilical cord (U-MSCs),[
Prosthetic devices [ Table 3 ]
Robotic exoskeletons or powered exoskeletons have emerged as an advantageous rehabilitation tool for certain disabled individuals with SCI. The studies provided preliminary evidence on efficacy of exoskeletons on cardiovascular health, energy expenditure, body composition, gait parameters, level of physical activity, neuropathic pain level, and quality of life. They can be used to restore a certain level of physical activity years after injury.[
CONCLUSION
We investigated the advancements in neuroprotective pharmacology, stem cell technologies, neuromodulation, and various external prosthetics for the treatment of SCI. However, more clinical trials and research will continue to establish their efficacy.
References
1. Ajiboye AB, Willett FR, Young DR, Memberg WD, Murphy BA, Miller JP. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: A proof-of-concept demonstration. Lancet. 2017. 389: 1821-30
2. Alexeeva N, Calancie B. Efficacy of quadropulse rTMS for improving motor function after spinal cord injury: Three case studies. J Spinal Cord Med. 2016. 39: 50-7
3. Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014. 137: 1394-409
4. Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016. 533: 247-50
5. Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med. 1990. 322: 1405-11
6. Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. National Acute Spinal Cord Injury Study. JAMA. 1997. 277: 1597-604
7. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2002. 3: CD001046-
8. Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain. 2012. 135: 1224-36
9. Cruciger O, Schildhauer TA, Meindl RC, Tegenthoff M, Schwenkreis P, Citak M. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: A case study. Disabil Rehabil Assist Technol. 2016. 11: 529-34
10. Dalamagkas K, Tsintou M, Seifalian AM. Stem cells for spinal cord injuries bearing translational potential. Neural Regen Res. 2018. 13: 35-42
11. Donati AR, Shokur S, Morya E, Campos DS, Moioli RC, Gitti CM. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep. 2016. 6: 30383-
12. Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016. 54: 8-15
13. Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma. 2011. 28: 787-96
14. Fehlings MG, Wilson JR, Frankowski RF, Toups EG, Aarabi B, Harrop JS. Riluzole for the treatment of acute traumatic spinal cord injury: Rationale for and design of the NACTN Phase I clinical trial. J Neurosurg Spine. 2012. 17: 151-6
15. Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006. 97: 1314-26
16. Geisler FH, Coleman WP, Grieco G, Poonian D, Sygen Study Group. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976). 2001. 26: S87-98
17. Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: A randomized. comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015. 29: 771-82
18. Gomes-Osman J, Field-Fote EC. Improvements in hand function in adults with chronic tetraplegia following a multi-day 10Hz rTMS intervention combined with repetitive task practice. J Neurol Phys Ther. 2015. 39: 23-
19. Gorgey AS. Robotic exoskeletons: The current pros and cons. World J Orthop. 2018. 9: 112-9
20. Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017. 92: 544-54
21. Grasmücke D, Zieriacks A, Jansen O, Fisahn C, Sczesny-Kaiser M, Wessling M. Against the odds: What to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Neurosurg Focus. 2017. 42: E15-
22. Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014. 31: 239-55
23. Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet. 2011. 377: 1938-47
24. Hentall ID, Gonzalez MM. Promotion of recovery from thoracic spinal cord contusion in rats by stimulation of medullary raphe or its midbrain input. Neurorehabil Neural Repair. 2012. 26: 374-84
25. Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012. 485: 372-5
26. Hofer AS, Schwab ME. Enhancing rehabilitation and functional recovery after brain and spinal cord trauma with electrical neuromodulation. Curr Opin Neurol. 2019. 32: 828-35
27. Hugenholtz H, Cass DE, Dvorak MF, Fewer DH, Fox RJ, Izukawa DM. High-dose methylprednisolone for acute closed spinal cord injury only a treatment option. Can J Neurol Sci. 2002. 29: 227-35
28. Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. 2000. 93: 1-7
29. James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018. 17: 905-17
30. Jin MC, Medress ZA, Azad TD, Doulames VM, Veeravagu A. Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurg Focus. 2019. 46: E10-
31. Kamiya K, Koda M, Furuya T, Kato K, Takahashi H, Sakuma T. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: A comparison with high-dose methylprednisolone as a historical control. Eur Spine J. 2015. 24: 963-7
32. Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C. Placebo-controlled study of rTMS combined with Lokomat® gait training for treatment in subjects with motor incomplete spinal cord injury. Exp Brain Res. 2016. 234: 3447-55
33. Li L, Adnan H, Xu B, Wang J, Wang C, Li F. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur Spine J. 2015. 24: 919-30
34. Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabil Neural Repair. 2016. 30: 951-62
35. Miller LE, Zimmermann AK, Herbert WG. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med Devices (Auckl). 2016. 9: 455-66
36. Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Arch Phys Med Rehabil. 2015. 96: S114-21
37. Pointillart V, Petitjean ME, Wiart L, Vital JM, Lassié P, Thicoipé M. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000. 38: 71-6
38. Raithatha R, Carrico C, Powell ES, Westgate PM, Chelette Ii KC, Lee K. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: A randomized pilot study. Neuro Rehabil. 2016. 38: 15-25
39. Saberi H, Firouzi M, Habibi Z, Moshayedi P, Aghayan HR, Arjmand B. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J Neurosurg Spine. 2011. 15: 515-25
40. Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci Lett. 2008. 443: 46-50
41. Schwartz G, Fehlings MG. Secondary injury mechanisms of spinal cord trauma: A novel therapeutic approach for the management of secondary pathophysiology with the sodium channel blocker riluzole. Prog Brain Res. 2002. 137: 177-90
42. Tabakow P, Raisman G, Fortuna W, Czyz M, Huber J, Li D. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014. 23: 1631-55
43. Takahashi H, Yamazaki M, Okawa A, Sakuma T, Kato K, Hashimoto M. Neuroprotective therapy using granulocyte colony-stimulating factor for acute spinal cord injury: A phase I/IIa clinical trial. Eur Spine J. 2012. 21: 2580-7
44. Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999. 19: 7037-47
45. Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013. 185: 485-92
46. Zhou XH, Ning GZ, Feng SQ, Kong XH, Chen JT, Zheng YF. Transplantation of autologous activated Schwann cells in the treatment of spinal cord injury: Six cases, more than five years of follow-up. Cell Transplant. 2012. 21: S39-47