- Department of Neurosurgery, Sestre milosrdnice University Hospital Center, Zagreb, Croatia,
- Department of Neurosurgery, Mostar University Hospital, Mostar, Bosnia and Herzegovina, Osijek, Croatia
- Department of Neurosurgery, Osijek University Hospital Center, Osijek, Croatia
- Department of Technology, Chair of Polymer Processing, Faculty of Mechanical Engineering and Naval Architecture, University of Zagreb, Zagreb, Croatia.
Correspondence Address:
Bruno Splavski, Department of Neurosurgery, Sestre milosrdnice University Hospital Center, Zagreb, Croatia.
DOI:10.25259/SNI_1239_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bruno Splavski1, Goran Lakicevic2, Marko Kovacevic3, Damir Godec4. Customized alloplastic cranioplasty of large bone defects by 3D-printed prefabricated mold template after posttraumatic decompressive craniectomy: A technical note. 22-Apr-2022;13:169
How to cite this URL: Bruno Splavski1, Goran Lakicevic2, Marko Kovacevic3, Damir Godec4. Customized alloplastic cranioplasty of large bone defects by 3D-printed prefabricated mold template after posttraumatic decompressive craniectomy: A technical note. 22-Apr-2022;13:169. Available from: https://surgicalneurologyint.com/surgicalint-articles/11546/
Abstract
Background: Manufacturing of customized three-dimensional (3D)-printed cranioplastic implant after decompressive craniectomy has been introduced to overcome the difficulties of intraoperative implant molding. The authors present and discuss the technique, which consists of the prefabrication of silicone implant mold using additive manufacturing, also known as 3D printing, and polymethyl methacrylate (PMMA) implant casting.
Methods: To reconstruct a large bone defect sustained after decompressive craniectomy due to traumatic brain injury (TBI), a 3D-printed prefabricated mold template was used to create a customized PMMA implant for cranial vault repair in five consecutive patients.
Results: A superb restoration of the symmetrical contours and curvature of the cranium was achieved in all patients. The outcome was clinically and cosmetically favorable in all of them.
Conclusion: Customized alloplastic cranioplasty using 3D-printed prefabricated mold for casting PMMA implant is easy to perform technique for the restoration of cranial vault after a decompressive craniectomy following moderate-to-severe TBI. It is a valuable and modern technique to advance manufacturing of personalized prefabricated cranioplastic implants used for the reconstruction of large skull defects having complex geometry. It is a safe and cost-effective procedure having an excellent cosmetic outcome, which may considerably decrease expenses and time needed for cranial reconstructive surgery.
Keywords: 3D-prited implant molding, Cranial vault restoration, Decompressive craniectomy, Traumatic brain injury
INTRODUCTION
Decompressive craniectomy is a surgical method for control of intracranial hypertension, which can be sustained by secondary brain damage following a severe traumatic brain injury (TBI).[
The autologous bone flap is frequently unavailable due to possible bone flap resorption and septic complications, which could lead to prolonged hospital stay and neurological deterioration, requiring a second surgery.[
Herein, we demonstrate and discuss a technique of cranial vault restoration after TBI decompressive craniectomy with the help of 3D customized molding of PMMA implant for alloplastic cranioplasty of large bone defects, contemplating its practical aspects, cosmetic effects, and safety.
MATERIALS AND METHODS
During the last couple of years, we have been involved in doing alloplastic cranioplasty by the help of additive manufacturing (3D printing). To repair a large/complex unilateral postcraniotomic bone defect sustained after a TBI, we have successfully used a 3D-printed prefabricated mold template to create a customized PMMA implant for bone repair in five consecutive patients.
All patients involved signed an informed consent allowing their personal data to be used for the purpose of this paper.
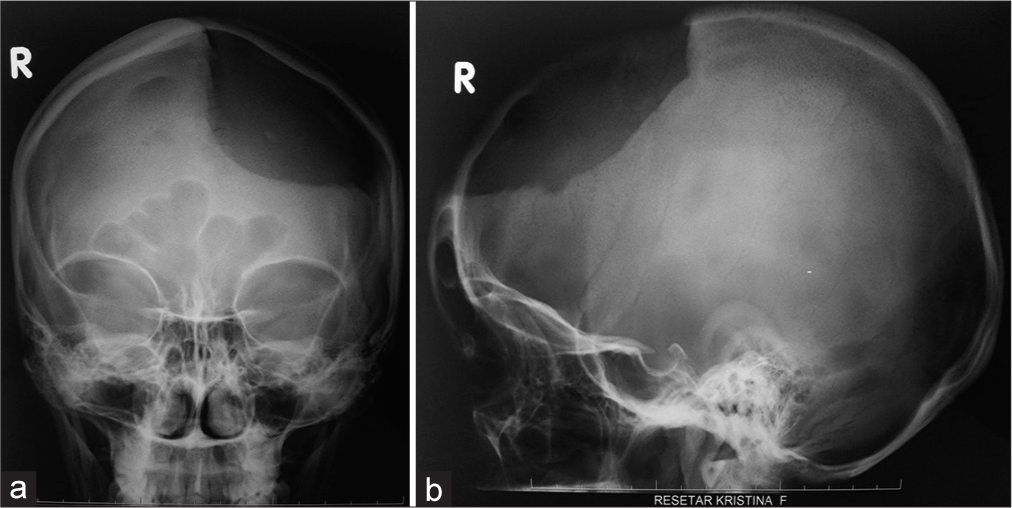
Preoperative plain X-rays and multisliced computerized tomography (MSCT) of the head were made in all patients before reconstructive surgery [
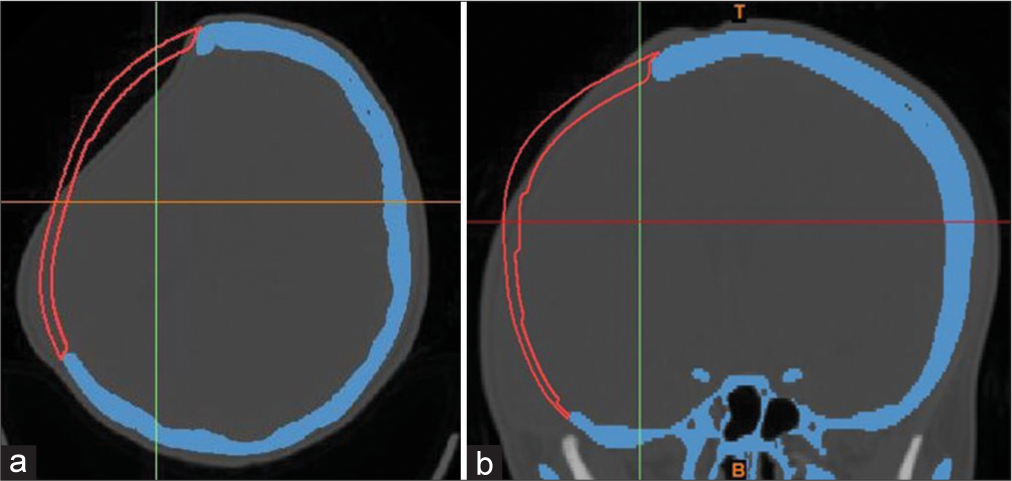
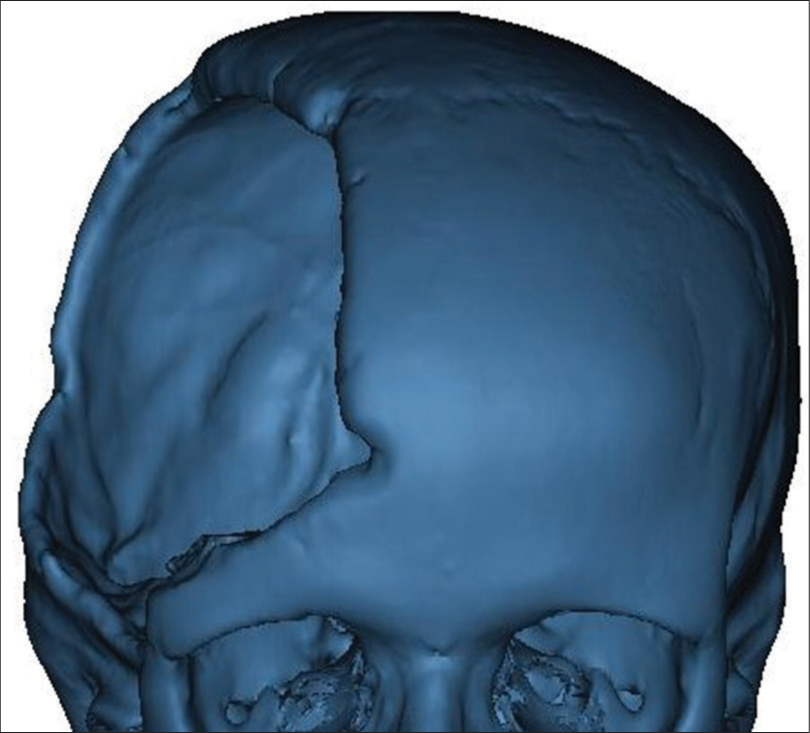
3D image of the skull was obtained from preoperative axial MSCT scan [
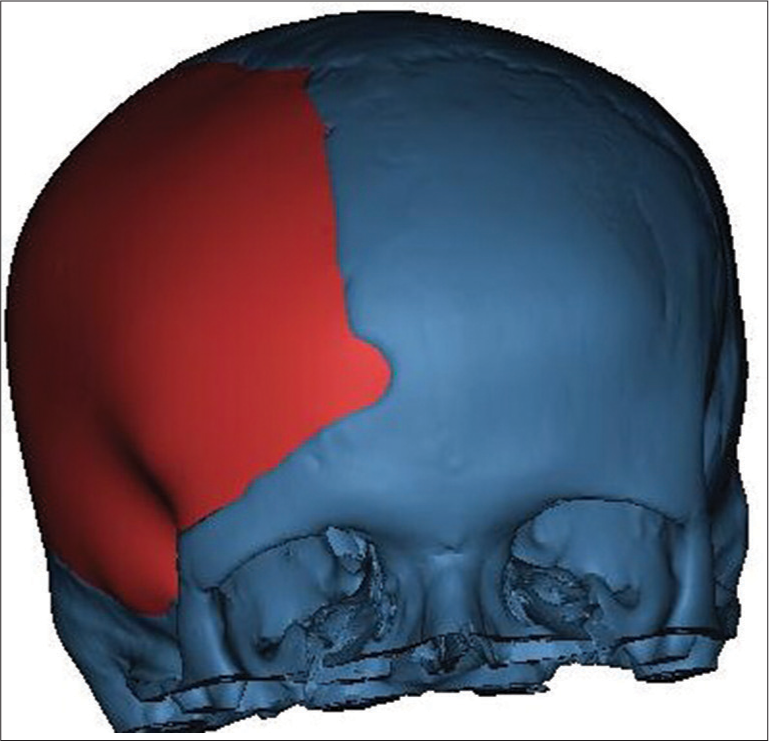
In the second phase, a customized 3D model of the implant was designed according to the initial images, which were employed to create an individualized prefabricated mold by the help of a PolyJet additive technology. The reason for designing a new model instead of using mirrored images was the natural asymmetry of the skull.
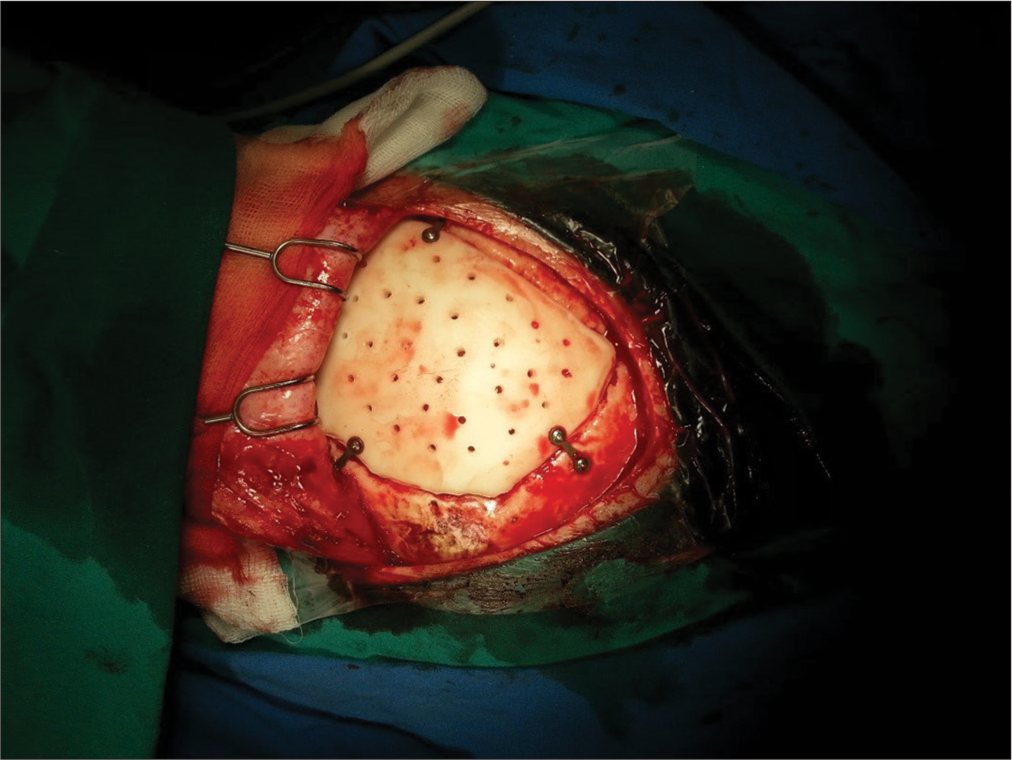
Synthetic mold was produced in a test center, sterilized, and brought to the operating room [
No prophylactic oral antibiotic covering was used peri- and postoperatively.
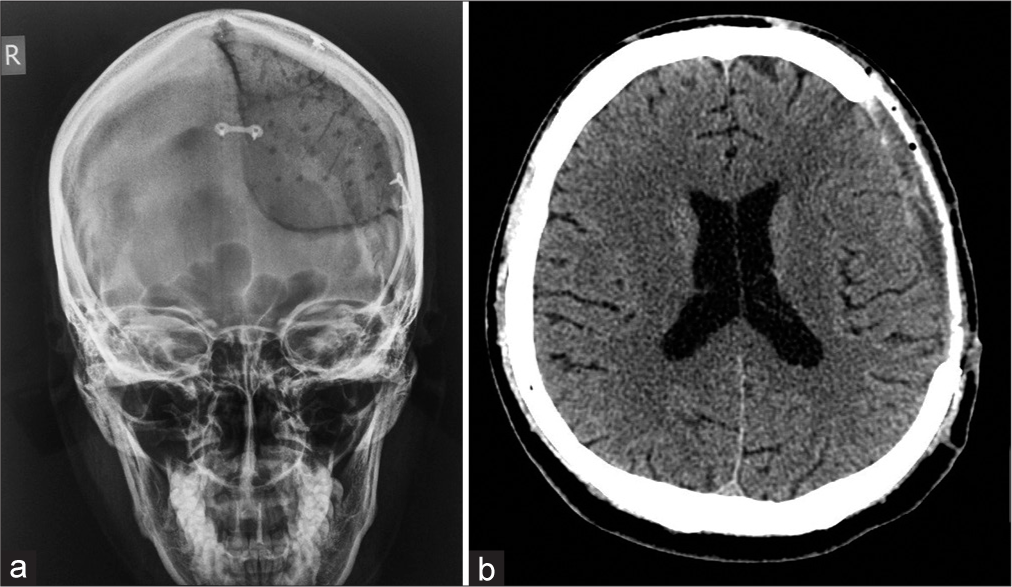
In all patients, plane cranial X-rays were performed immediately postsurgery to check the implant’s position and contours [
Patients’ personal satisfaction with the outcome of reconstructive surgery was assessed on regular follow-ups too.
RESULTS AND DISCUSSION
Five patients with large one-sided frontoparietal- temporal skull defects (>100 cm2) underwent alloplastic PMMA cranioplasty over a 6-year period (2014–2020) [
The group consisted of three females and two males, ranging in age between 21 and 57 years. The mean patients’ age at the time of cranioplasty was 37.3 years.
The median surgical time for cranioplasty was 96 ± 17 min, while the mean duration of the intraoperative molding process was 21 ± 8 min.
Postoperative X-rays and MSCT head scans showed excellent restoration of symmetrical cranial contours and curvature, in addition to well-fitting implants in all patients [
Patients were followed up in the period between 5 and 1 year postcranioplasty with careful assessment of their clinical status, morphological appearance, and personal satisfaction. The median follow-up period was 25 months (ranging 12–28 months).
Postoperative infection developed in one patient who developed an open wound defect postcranioplasty. This patient underwent a repeated late PMMA cranioplasty after the infected implant removal and consecutive broad antibiotic treatment, leading to complete wound healing. The time elapsed between the two cranioplasties was 9 months.
The outcome was clinically favorable and cosmetically excellent in all patients. All were very satisfied with the result of reconstructive surgery and felt comfortable with their final cosmetic effect.
In this paper, we discussed the use of 3D printing additive technology for the construction of a mold by which a PMMA implant for the restoration of huge/complex unilateral skull bone defect was produced. We also addressed the practical advantages and pitfalls of the procedure, comparing it to other contemporary cranioplastic techniques. Finally, we reviewed the literature and debated over future perspectives of the technology.
Reconstruction of a cranial vault is particularly demanding surgical procedure.[
Cranioplasty with an autologous bone flap after decompressive craniectomy has been the preferable treatment for cranial reconstruction.[
However, implant’s design and materials are not standardized yet. Different materials with different mechanical properties dissimilar to that of the lost bone at the site of implantation are in use currently.[
In this series, we have opted for an intraoperative PMMA implant molding based on the use of prefabricated 3D-prited mold where PolyJet additive manufacturing technology was employed. It consists of jetting a thin layer of photosensitive polymer material in a form of fine droplets, followed by curing with a source of ultraviolet light.[
Considering the above-mentioned advantages of the technique, we believe that a personalized prefabrication of the mold template to produce a PMMA cranioplastic implant is more effective and less costly technique than previously reported use of other tailored cranial implants,[
Nevertheless, further advances in virtual reality, additive manufacturing, and 3D image-based reconstruction practice will result in even faster data processing and manufacturing of more demanding, ideally adjusted cranioplastic implants.[
Seeing our results and remembering the paper limitations arising from small number of patients in this series, supplementary research is needed to confirm practical applicability of this technique on a broader setting.
CONCLUSION
The 3D printing technology is a valuable and modern technique to advance manufacturing of personalized prefabricated cranioplastic implants used for the reconstruction of large skull defects having complex geometry.
Customized alloplastic cranioplasty using a 3D-printed prefabricated mold for casting PMMA implant is a safe and easy to use bone defect reconstruction technique after decompressive craniectomy following TBI. It ensures a favorable clinical outcome and excellent cosmetic effect as well as decreases the time needed for reconstructive surgery and the risk of postoperative complications.
Authors’ contributions
All coauthors were included in substantial contribution to conception and design and final approval of the version to be published.
Ethical approval
The research reported in submitted paper has been conducted in an ethical and responsible manner and is in full compliance with all relevant codes legislation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abdel Hay J, Smayra T, Moussa R. Customized polymethylmethacrylate cranioplasty implants using 3-dimensional printed polylactic acid molds: Technical note with 2 illustrative cases. World Neurosurg. 2017. 105: 971-9
2. Balossier A, Durand A, Achim VV, Noudel R, Hurel S, Emery E. Reconstruction of the cranial vault using CAD/ CAM-fabricated glass bioceramic implants. Neurochirurgie. 2011. 57: 21-7
3. Basu B, Bhaskar N, Barui S, Sharma V, Das S, Govindarajan N. Evaluation of implant properties, safety profile and clinical efficacy of patient-specific acrylic prosthesis in cranioplasty using 3D binderjet printed cranium model: A pilot study. J Clin Neurosci. 2021. 85: 132-42
4. Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009. 26: E10
5. Camarini ET, Tomeh JK, Dias RR, da Silva EJ. Reconstruction of frontal bone using specific implant polyether-ether-ketone. J Craniofac Surg. 2011. 22: 2205-7
6. Chacón-Moya E, Gallegos-Hernández JF, Piña-Cabrales S, Cohn-Zurita F, Goné-Fernández A. Cranial vault reconstruction using computer-designed polyetheretherketone (PEEK) implant: Case report. Cir Cir. 2009. 77: 437-40
7. Chamo D, Msallem B, Sharma N, Aghlmandi S, Kunz C, Thieringer FM. Accuracy assessment of molded, patient-specific polymethylmethacrylate craniofacial implants compared to their 3D printed originals. J Clin Med. 2020. 9: 832
8. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
9. Cheng CH, Chuang HY, Lin HL, Liu CL, Yao CH. Surgical results of cranioplasty using three-dimensional printing technology. Clin Neurol Neurosurg. 2018. 168: 118-23
10. da Silva EB, de Aragão AH, de Paula Loureiro M, Lobo CS, Oliveti AF, de Oliveira RM. Cranioplasty with three-dimensional customised mould for polymethylmethacrylate implant: A series of 16 consecutive patients with cost-effectiveness consideration. 3D Print Med. 2021. 7: 4
11. Dean D, Min KJ, Bond A. Computer aided design of large-format prefabricated cranial plates. J Craniofac Surg. 2003. 14: 819-32
12. De La Peña A, De La Peña-Brambila J, Pérez-De La Torre J, Ochoa M, Gallardo GJ. Low-cost customized cranioplasty using a 3D digital printing model: A case report. 3D Print Med. 2018. 4: 4
13. Desai JB. Cost-effective technique of fabrication of polymethyl methacrylate based cranial implant using three-dimensional printed moulds and wax elimination technique. J Craniofac Surg. 2019. 30: 1259-63
14. Dobran M, Nasi D, Polonara G, Paracino R, Mancini F, Costanza MD. Clinical and radiological risk factors of autograft cranioplasty resorption after decompressive craniectomy for traumatic brain injury. Clin Neurol Neurosurg. 2020. 196: 105979
15. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: Cosmetic or therapeutic?. Surg Neurol. 1997. 47: 238-41
16. Eppley BL, Kilgo M, Coleman JJ. Cranial reconstruction with computer-generated hard-tissue replacement patient-matched implants: Indications, surgical technique, and long-term follow-up. Plast Reconstr Surg. 2002. 109: 864-71
17. Gebhardt A.editors. Understanding Additive Manufacturing. München: Carl Hanser Verlag; 2012. p.
18. Gerbino G, Zavattero E, Zenga F, Bianchi FA, Garzino-Demo P, Berrone S. Primary and secondary reconstruction of complex craniofacial defects using polyetheretherketone custom-made implants. J Craniomaxillofac Surg. 2015. 43: 1356-63
19. Godec D, Šercer M.editors. Additive Manufacturing. Zagreb: University of Zagreb; 2015. p.
20. Goh RC, Chang CN, Lin CL, Lo LJ. Customised fabricated implants after previous failed cranioplasty. J Plast Reconstr Aesthet Surg. 2010. 63: 1479-84
21. Gopal S, Rudrappa S, Sekar A, Preethish-Kumar V, Masapu D. Customized and cost-effective 3D printed mold for cranioplasty: India’s first single center experience. Neurol India. 2021. 69: 611-7
22. Göttsche J, Mende KC, Schram A, Westphal M, Amling M, Regelsberger J. Cranial bone flap resorption-pathological features and their implications for clinical treatment. Neurosurg Rev. 2021. 44: 2253-60
23. Hieu LC, Bohez E, Vander Sloten J, Oris P, Phien HN, Vatcharaporn E. Design and manufacturing of cranioplasty implants by 3-axis CNC milling. Technol Health Care. 2002. 10: 413-23
24. Hoffmann B, Sepehrnia A. Tailored implants for alloplastic cranioplasty--clinical and surgical considerationsm. Acta Neurochir (Wien). 2005. 93: 127-9
25. Iaccarino C, Kolias A, Adelson PD, Rubiano AM, Viaroli E, Buki A. Consensus statement from the international consensus meeting on post-traumatic cranioplasty. Acta Neurochir (Wien). 2021. 163: 423-40
26. Iaccarino C, Kolias AG, Roumy LG, Fountas K, Adeleye AO. Cranioplasty following decompressive craniectomy. Front Neurol. 2020. 10: 1357
27. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 2003. 5: 591-6
28. Kim BJ, Hong KS, Park KJ, Park DH, Chung YG, Kang SH. Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J Korean Neurosurg Soc. 2012. 52: 541-6
29. Korhonen TK, Salokorpi N, Niinimäki J, Serlo W, Lehenkari P, Tetri S. Quantitative and qualitative analysis of bone flap resorption in patients undergoing cranioplasty after decompressive craniectomy. J Neurosurg. 2018. 130: 312-21
30. Lannon M, Algird A, Alsunbul W, Wang BH. Cost-effective cranioplasty utilizing 3D printed molds: A Canadian single-center experience. Can J Neurol Sci. 2021. 5: 1-7
31. Lee SC, Wu CT, Lee ST, Chen PJ. Cranioplasty using polymethyl methacrylate prostheses. J Clin Neurosci. 2009. 16: 56-63
32. Marbacher S, Andereggen L, Erhardt S, Fathi AR, Fandino J, Raabe A. Intraoperative template-molded bone flap reconstruction for patient-specific cranioplasty. Neurosurg Rev. 2012. 35: 527-35
33. Mavili ME, Canter HI, Saglam-Aydinatay B, Kamaci S, Kocadereli I. Use of three-dimensional medical modeling methods for precise planning of orthognathic surgery. J Craniofac Surg. 2007. 18: 740-7
34. Morales-Gómez JA, Garcia-Estrada E, Leos-Bortoni JE, Delgado-Brito M, Flores-Huerta LE, De La Cruz-Arriaga AA. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J Neurosurg. 2018. 15: 1-7
35. Muzevic D, Splavski B. The Lund concept for severe traumatic brain injury. Cochrane Database Syst Rev. 2013. 12: 1-13
36. Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014. 4: 9-18
37. Rotaru H, Stan H, Florian IS, Schumacher R, Park YT, Kim SG. Cranioplasty with custom-made implants: analyzing the cases of 10 patients. J Oral Maxillofac Surg. 2012. 70: e169-76
38. Saringer W, Nöbauer-Huhmann I, Knosp E. Cranioplasty with individual carbon fibre reinforced polymere (CFRP) medical grade implants based on CAD/CAM technique. Acta Neurochir (Wien). 2002. 144: 1193-203
39. Schebesch KM, Höhne J, Gassner HG, Brawanski A. Preformed titanium cranioplasty after resection of skull base meningiomas a technical note. J Craniomaxillofac Surg. 2013. 41: 803-7
40. Schön SN, Skalicky N, Sharma N, Zumofen DW, Thieringer FM. 3D-printer-assisted patient-specific polymethyl methacrylate cranioplasty: A case series of 16 consecutive patients. World Neurosurg. 2021. 148: e356-62
41. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994. 34: 729-31
42. Shim JH, Yoon MC, Jeong CM, Jang J, Jeong SI, Cho DW. Efficacy of rhBMP-2 loaded PCL/PLGA/β-TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed Mater. 2014. 9: 65006
43. Siracusa V, Maimone G, Antonelli V. State-of-art of standard and innovative materials used in cranioplasty. Polymers (Basel). 2021. 13: 1452
44. Staffa G, Barbanera A, Faiola A, Fricia M, Limoni P, Mottaran R. Custom made bioceramic implants in complex and large cranial reconstruction: A two-year follow-up. J Craniomaxillofac Surg. 2012. 40: e65-70
45. Tan ET, Ling JM, Dinesh SK. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J Neurosurg. 2016. 124: 1531-7
46. Vlok AJ, Naidoo S, Kamat AS, Lamprecht D. Evaluation of locally manufactured patient-specific custom made implants for cranial defects using a silicone mould. S Afr J Surg. 2018. 56: 38-42
47. Winter CD, Adamides A, Rosenfeld JV. The role of decompressive craniectomy in the management of traumatic brain injury: A critical review. J Clin Neurosci. 2005. 12: 619-23
48. Wulf J, Busch LC, Golz T, Knopp U, Giese A, Ssenyonjo H. CAD generated mold for preoperative implant fabrication in cranioplasty. Stud Health Technol Inform. 2005. 111: 608-10
49. Yang J, Sun T, Yuan Y, Li X, Zhou Y, Guan J. Risk factors for bone flap resorption after autologous bone cranioplasty: Protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2020. 99: e21035