- Department of Neurology, University of Toronto, Toronto, Canada,
- Department of Neurology, University of Iowa, Iowa City, Iowa,
- Department of Neurology, University of New Mexico, Albuquerque, New Mexico,
- Department of Neurology, University of Missouri, Columbia, Missouri,
- Department of Neurology, University of Tennessee, Memphis, Tennessee,
- Department of Neurology, Massachusetts General Hospital, Boston, Massachusetts, United States.
Correspondence Address:
Atif Zafar, Department of Neurology, University of Toronto, Toronto, Canada, Canada, United States.
DOI:10.25259/SNI_569_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Atif Zafar1, Mudassir Farooqui2, Asad Ikram3, Sajid Suriya3, Duraisamy Kempuraj4, Mohammad Khan5, Nudrat Tasneem2, Dania Qaryouti3, Syed Quadri6, Harold P. Adams2, Santiago Ortega-Gutierrez2, Enrique Leira2, Asgar Zaheer4. Cytokines, brain proteins, and growth factors in acute stroke patients: A pilot study. 27-Jul-2021;12:366
How to cite this URL: Atif Zafar1, Mudassir Farooqui2, Asad Ikram3, Sajid Suriya3, Duraisamy Kempuraj4, Mohammad Khan5, Nudrat Tasneem2, Dania Qaryouti3, Syed Quadri6, Harold P. Adams2, Santiago Ortega-Gutierrez2, Enrique Leira2, Asgar Zaheer4. Cytokines, brain proteins, and growth factors in acute stroke patients: A pilot study. 27-Jul-2021;12:366. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11007
Abstract
Background: Immunomodulation and cell signaling involve several cytokines, proteins, and other mediators released in response to the trauma, inflammation, or other insults to the central nervous system. This pilot study is part of the registry designed to evaluate the temporal trends among these molecules after an acute ischemic stroke (AIS) in patients.
Methods: Twelve AIS patients were enrolled within 24 hours of the symptoms onset. Two sets of plasma samples were collected: First at admission and second at 24 hours after admission. Cytokines/chemokines and other inflammatory molecules were measured using multiplex assay kit.
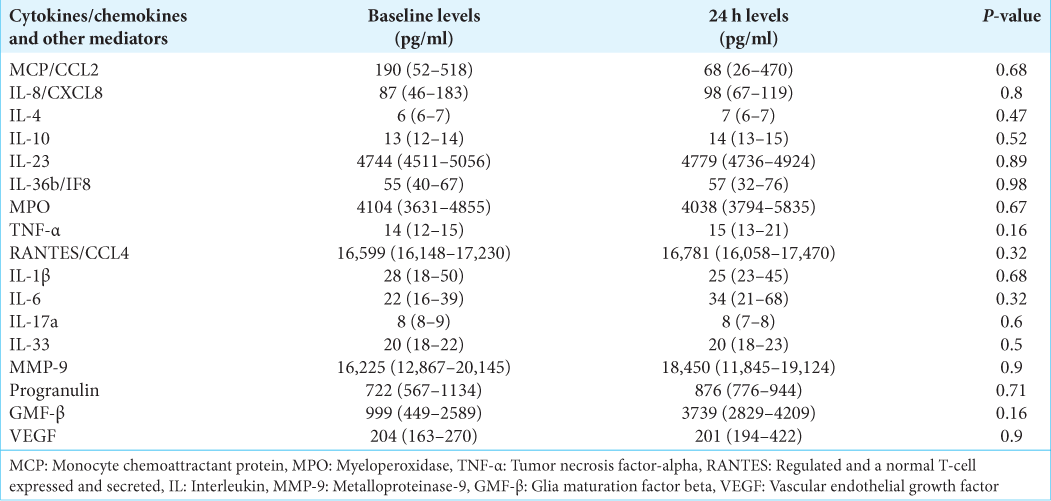
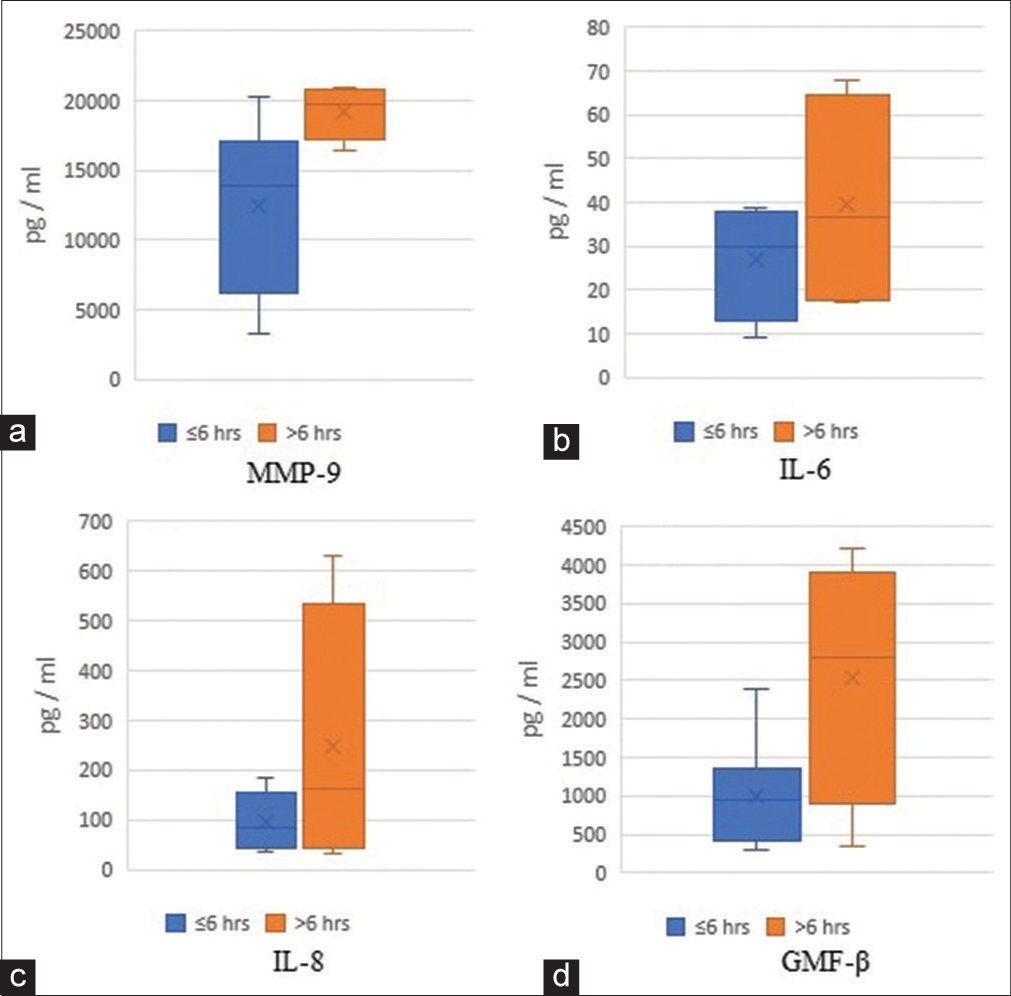
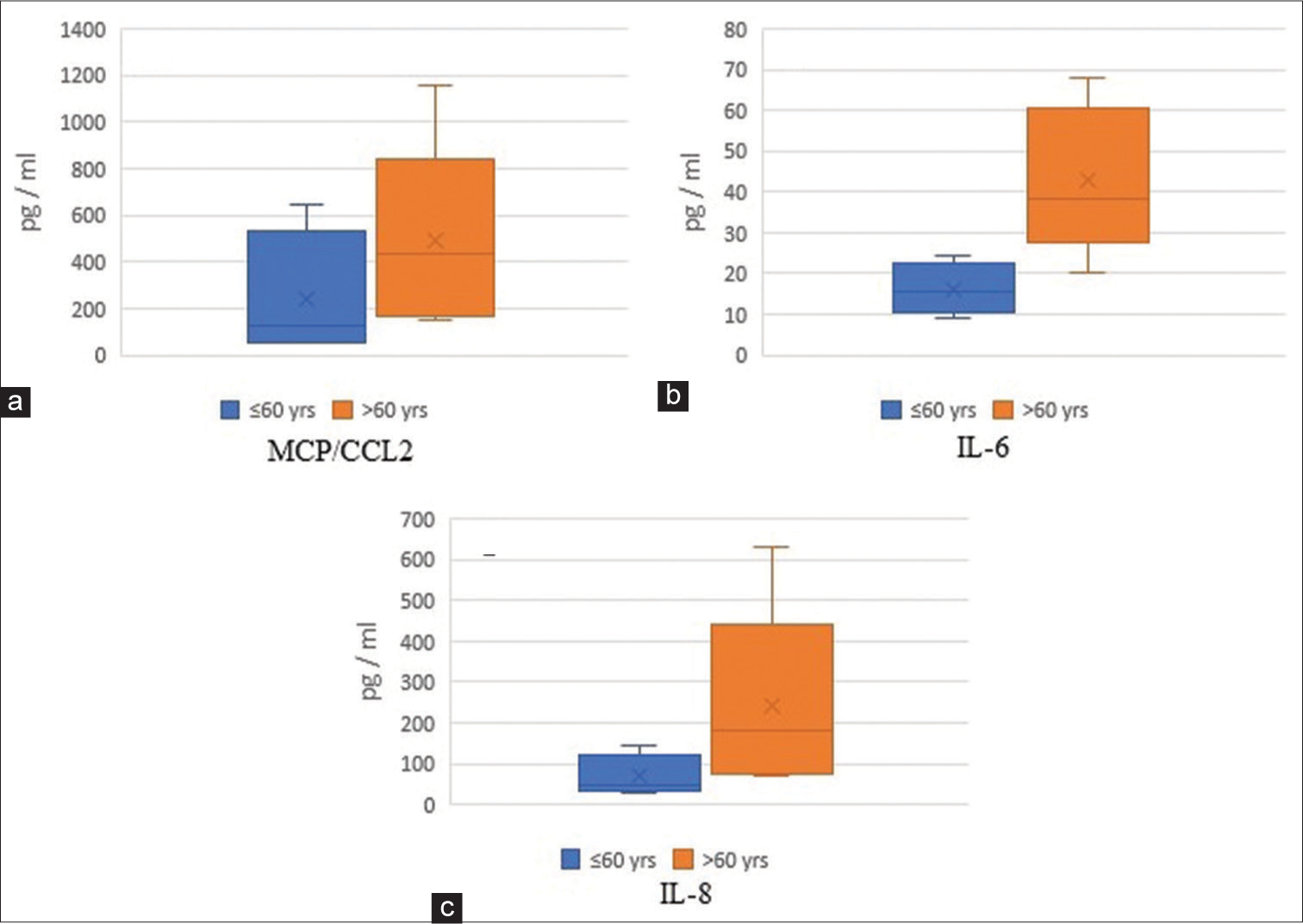
Results: An increased trend in IL-6 (22 vs. 34 pg/ml), IL-8/CXCL8 (87 vs. 98 pg/ml), MMP-9 (16225 vs. 18450 pg/ml), and GMF-β (999 vs. 3739 pg/ml) levels was observed overtime after an AIS. Patients ≤60 years had lower levels of plasma MCP-1/CCL2 (50–647 vs. 150–1159 pg/ml), IL-6 (9–25 vs. 20–68 pg/ml), and IL-8 (30– 143 vs. 72–630 pg/ml), when compared with patients >60 years old.
Conclusion: Cytokines/chemokines and other inflammatory mediators play an important role in the pathogenesis of stroke in addition to mediating poststroke inflammation. Further research is needed to evaluate and characterize the cumulative trends of these mediators for the clinical prognosis or as surrogate biomarkers.
Keywords: Acute ischemic brain injury, Cytokine, Interleukin, Poststroke inflammation, Stroke
INTRODUCTION
In the United States (US), stroke is the fifth leading cause of mortality, affecting approximately 0.8 million people every year.[
MATERIALS AND METHODS
Twelve consecutive adult (>18 years) AIS patients presenting within 24 hours of the onset of symptoms were consented for this institutional (University of Iowa, Iowa City, IA) review board (IRB) approved study in accordance with the ethical standards of the Declaration of Helsinki. Blood samples were collected at the time of admission and after 24 hours. Exclusion criteria were; prior stroke or any other neurodegenerative or neuroinflammatory disease (Alzheimer’s disease, Parkinson’s disease [PD], multiple sclerosis [MS], etc.), acute infection, chronic inflammatory systemic illness, pregnancy, use of immunosuppressive medications or steroids, and a premorbid modified Rankin score ≥5. Diagnosis of IS was based on clinical and radiological evidence at the discretion of the stroke neurologist. Patients clinical characteristics including stroke etiology and stroke severity were collected.[
RESULTS
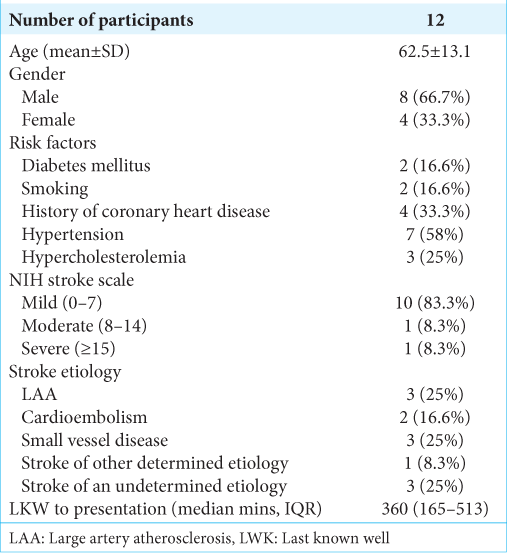
Patient’s demographics, stroke etiology, and severity are described. [
DISCUSSION
Our pilot study observed an increasing trend overtime in IL-6, IL-8, MMP-9, and GMF-β levels after an AIS. We also observed that MCP/CCL2, IL-6, and IL-8 levels were increased among older AIS patients (>60 years).
This is the first report characterizing GMF-β levels in AIS patients. GMF-β is a pro-inflammatory protein expressed in the brain, especially in neurons, microglia, and astrocytes.[
We also observed increased levels of acute-phase pro-inflammatory cytokines, IL-6, IL-8, and MCP-1/CCL2. IL-6 is an endogenous pyrogen induced by IL-2, IL-4, prostaglandins, and interferon-gamma, while IL-8 is induced by macrophages and MCP-1/CCL2 by microglia/macrophages, endothelial cells, astrocytes, and neurons; acting as a chemoattractant for neutrophils, helping recruit inflammatory cells to the site of injury during the early phase inflammatory response. Studies have reported increased IL-6 and IL-8 levels as early as 1–4 hours after ischemic insult and remain elevated for the next 24–72 hours.[
These observations corroborate the role of these pro- and anti-inflammatory cytokines and other mediators in AIS patients, however, prognostication and clinical utility of these cytokines in predicting functional outcome, stroke types, and mortality are still debatable. Cytokines/chemokines constitute part of a complex immune system. Disruption in the intricate pathway implies downstream modulation on the dynamic feedback circuitry. As we expand our understanding of these molecular interactions, there still remains a lack of knowledge concerning integrative molecular alterations and their impact. The proposed comprehensive database comprising clinical, imaging, and molecular data would help expand our understanding and elucidate molecules that are systemically altered in AIS patients. “Cytokine Registry In Stroke Patients,” NCT03297827 was established to study the role of these cytokines and molecules and their trend variations in stroke patients, eventually leading to develop new therapeutic and management strategies.
CONCLUSION
This preliminary study reports the trends in GMF-β, a pro-inflammatory protein in AIS patients. We also corroborated findings of acute-phase inflammatory cytokines/chemokines and protein, including IL-6, IL-8, MCP-1/CCL2, and MMP-9 during the early phase after an acute ischemic injury.
Clinical trial registration
URL: http://www.clinicaltrials.gov Unique identifier: NCT03297827.
Cytokine Registry In Stroke Patients (CRISP) trial.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Stoppelmoor – Adams Stroke Education and Research Fund.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL. Classification of subtype of acute ischemic stroke, Definitions for use in a multicenter clinical trial, TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993. 24: 35-41
2. Ahmed ME, Selvakumar GP, Kempuraj D, Raikwar SP, Thangavel R, Bazley K. Glia maturation factor (GMF) regulates microglial expression phenotypes and the associated neurological deficits in a mouse model of traumatic brain injury. Mol Neurobiol. 2020. 57: 4438-50
3. Ahmed ME, Selvakumar GP, Kempuraj D, Raikwar SP, Thangavel R, Bazley K. Neuroinflammation mediated by glia maturation factor exacerbates neuronal injury in an in vitro model of traumatic brain injury. J Neurotrauma. 2020. 37: 1645-55
4. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation. 2018. 137: e67-492
5. Bonifačić D, Toplak A, Benjak I, Tokmadžić VS, Lekić A, Kučić N. Monocytes and monocyte chemoattractant protein 1 (MCP-1) as early predictors of disease outcome in patients with cerebral ischemic stroke. Wien Klin Wochenschr. 2016. 128: 20-7
6. Castellanos M, Sobrino T, Millán M, García M, Arenillas J, Nombela F. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: A multicenter confirmatory study. Stroke. 2007. 38: 1855-9
7. Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K. Inflammatory markers and onset of cardiovascular events: Results from the health ABC study. Circulation. 2003. 108: 2317-22
8. Fan J, Fong T, Chen X, Chen C, Luo P, Xie H. Glia maturation factor-β: A potential therapeutic target in neurodegeneration and neuroinflammation. Neuropsychiatr Dis Treat. 2018. 14: 495-504
9. Farooqui M, Ikram A, Suriya S, Saleem S, Quadri SA, Robinson M. Cytokine registry in stroke patients (CRISP): Protocol of a prospective observational study. Medicine (Baltimore). 2020. 99: e20921
10. Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: Kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994. 122: 135-9
11. Georgakis MK, Malik R, Björkbacka H, Pana TA, Demissie S, Ayers C. Circulating monocyte chemoattractant protein-1 and risk of stroke: Meta-analysis of population-based studies involving 17 180 individuals. Circ Res. 2019. 125: 773-82
12. Jenny NS, Callas PW, Judd SE, McClure LA, Kissela B, Zakai NA. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology. 2019. 92: e2375-84
13. Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Kumar KK. Glia maturation factor and mast cell-dependent expression of inflammatory mediators and proteinase activated receptor-2 in neuroinflammation. J Alzheimers Dis. 2018. 66: 1117-29
14. Kostulas N, Pelidou SH, Kivisäkk P, Kostulas V, Link H. Increased IL-1β, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. 1999. 30: 2174-9
15. Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012. 32: 1677-98
16. Lim R, Liu YX, Zaheer A. Cell-surface expression of glia maturation factor beta in astrocytes. FASEB J. 1990. 4: 3360-3
17. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): A phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017. 16: 781-8
18. Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004. 35: 57-63
19. Montaner J, Alvarez-Sabín J, Molina C, Anglés A, Abilleira S, Arenillas J. Matrix metalloproteinase expression after human cardioembolic stroke: Temporal profile and relation to neurological impairment. Stroke. 2001. 32: 1759-66
20. Ning M, Furie K, Koroshetz W, Lee H, Barron M, Lederer M. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006. 66: 1550-5
21. Ormstad H, Aass HC, Lund-Sørensen N, Amthor KF, Sandvik L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011. 258: 677-85
22. Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: A systematic review. J Stroke Cerebrovasc Dis. 2011. 20: 47-54
23. Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK.editors. Early Biomarkers of Stroke. United Kingdom: Oxford University Press; 2003. p.
24. Rosell A, Cuadrado E, Ortega-Aznar A, HernándezGuillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina Type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008. 39: 1121-6
25. Selvakumar GP, Ahmed ME, Iyer SS, Thangavel R, Kempuraj D, Raikwar SP. Absence of glia maturation factor protects from axonal injury and motor behavioral impairments after traumatic brain injury. Exp Neurobiol. 2020. 29: 230-48
26. Suriya S, Farooqui M, Ikram A, Qaryouti D, Quadri S, Gutierrez SO.editors. Role of Cytokines in Acute Ischemic Stroke Patients: Preliminary Analysis of a Prospective Database (5521), AAN Enterprises. 2020. p.
27. Vila N, Castillo J, Dávalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000. 31: 2325-9
28. Wang BR, Zaheer A, Lim R. Polyclonal antibody localizes glia maturation factor beta-like immunoreactivity in neurons and glia. Brain Res. 1992. 591: 1-7
29. Zafar A, Ikram A, Jillella DV, Kempuraj D, Khan MM, Bushnaq S. Measurement of elevated IL-37 levels in acute ischemic brain injury: A cross-sectional pilot study. Cureus. 2017. 9: e1767
30. Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN. A novel role of glia maturation factor: Induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem. 2007. 101: 364-76