- Department of Neurosurgery, Tsukuba Memorial Hospital, Tsukuba, Ibaraki, Japan.
- Department of Radiology, Tsukuba Memorial Hospital, Tsukuba, Ibaraki, Japan.
- Department of Neurology, University of Tsukuba, Tsukuba, Ibaraki, Japan.
- Department of Neurosurgery, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, University of Tsukuba, Tsukuba, Ibaraki, Japan.
DOI:10.25259/SNI_274_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Michihide Kajita1, Kiyoyuki Yanaka1, Sho Hanai1, Hitoshi Aiyama1, Nobuyuki Takahashi2, Shinji Saiki3, Eiichi Ishikawa4. De novo formation of twig-like middle cerebral artery: An illustrative case. 02-Jun-2023;14:192
How to cite this URL: Michihide Kajita1, Kiyoyuki Yanaka1, Sho Hanai1, Hitoshi Aiyama1, Nobuyuki Takahashi2, Shinji Saiki3, Eiichi Ishikawa4. De novo formation of twig-like middle cerebral artery: An illustrative case. 02-Jun-2023;14:192. Available from: https://surgicalneurologyint.com/surgicalint-articles/12344/
Abstract
Background: Twig-like middle cerebral artery (T-MCA) is a rare vascular abnormality characterized by the replacement of the M1 segment of the middle cerebral artery (MCA) with a plexiform arterial network of small vessels. T-MCA is generally regarded as an embryological persistence. Conversely, T-MCA may also be a secondary sequela but no reports of cases of de novo formation exist. Here, we report the first case describing possible de novo T-MCA formation.
Case Description: A 41-year-old woman was referred to our hospital from a nearby clinic because of transient left hemiparesis. Magnetic resonance (MR) imaging revealed mild stenosis of the bilateral MCAs. The patient then underwent MR imaging follow-ups once a year. MR imaging at the age of 53 showed a right M1 occlusion. Cerebral angiography revealed a right M1 occlusion and formation of a plexiform network consistent with the occlusion site, leading to the diagnosis of de novo T-MCA.
Conclusion: This is the first case report describing possible de novo T-MCA formation. Although a detailed laboratory examination did not confirm the etiology, autoimmune disease was suspected to have precipitated this vascular lesion.
Keywords: Anomaly, Autoimmune disease, Etiology, Middle cerebral artery, Twig
INTRODUCTION
Twig-like middle cerebral artery (T-MCA) is a rare vascular anomaly characterized by the replacement of the M1 MCA segment with a plexiform network of small vessels.[
It is generally believed that the etiology of T-MCA results from an embryonic abnormality in proximal MCA development. Although some investigators have suggested that T-MCA may be acquired,[
CASE DESCRIPTION
A 41-year-old woman presented to her primary care physician after experiencing one episode of left upper and lower extremity weakness lasting several minutes. Because her father had been diagnosed with MMD, the patient was referred to our hospital in 2011 for a close examination of her cerebrovascular system due to possible familial occurrence of MMD.
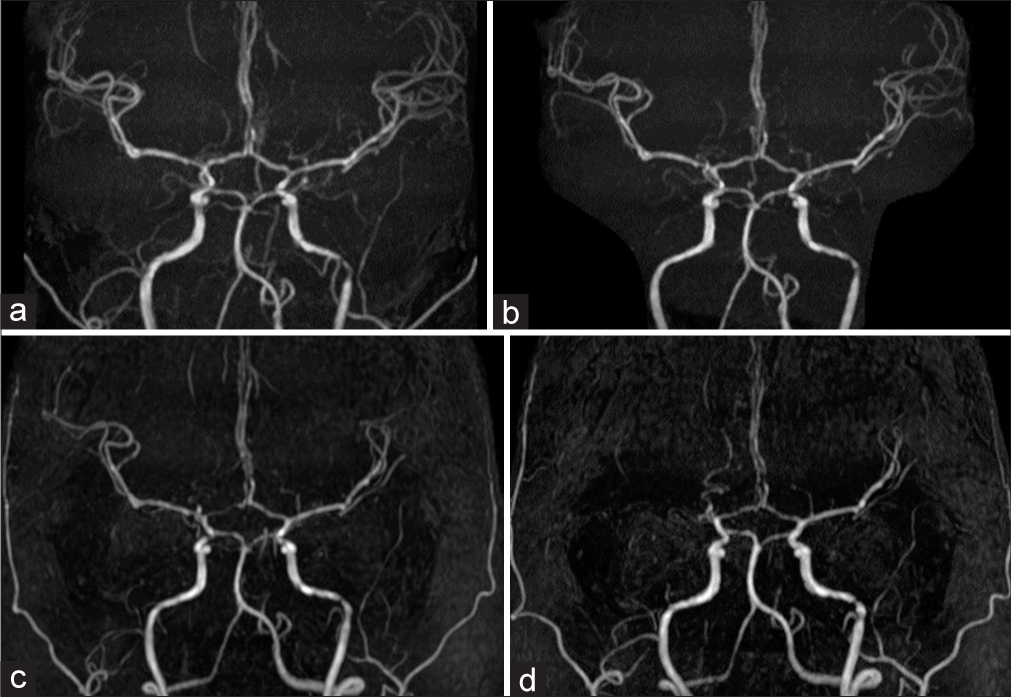
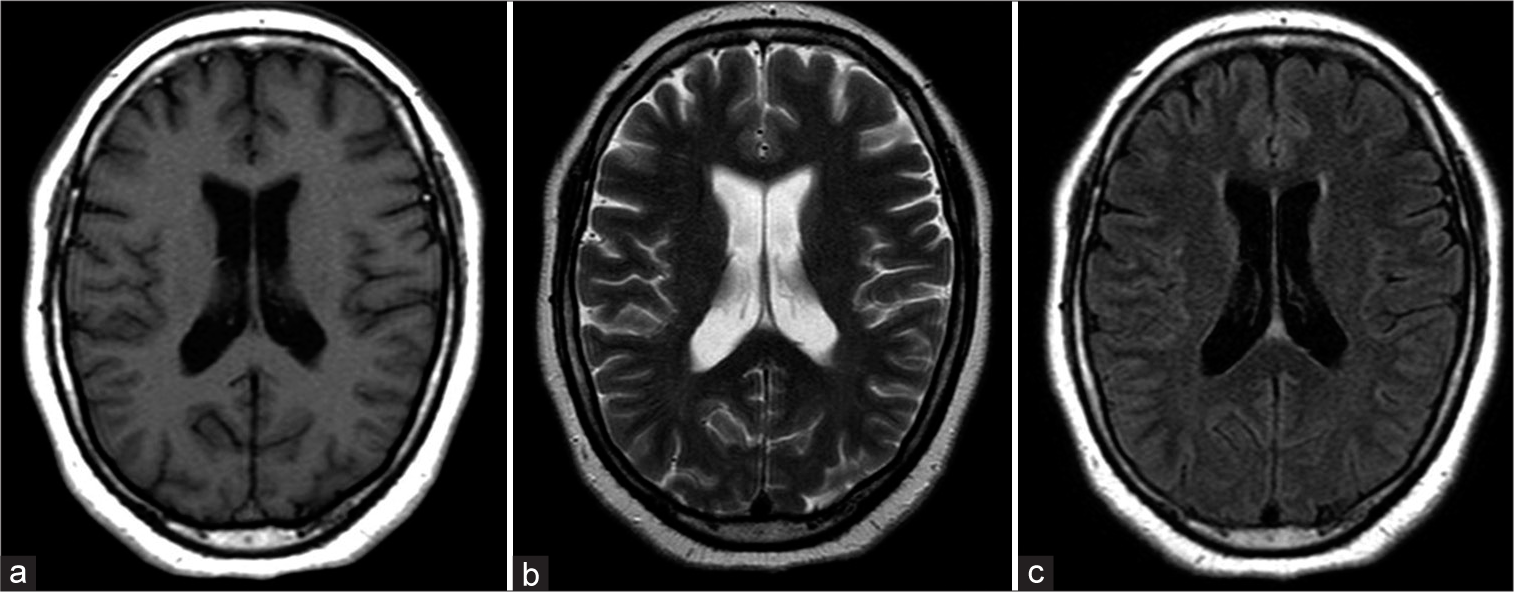
The patient was asymptomatic at the initial presentation. Based on initial magnetic resonance imaging (MRI), mild stenosis in the bilateral MCA was suspected but there was neither stenosis in the ICA and no evidence of moyamoya vessels [
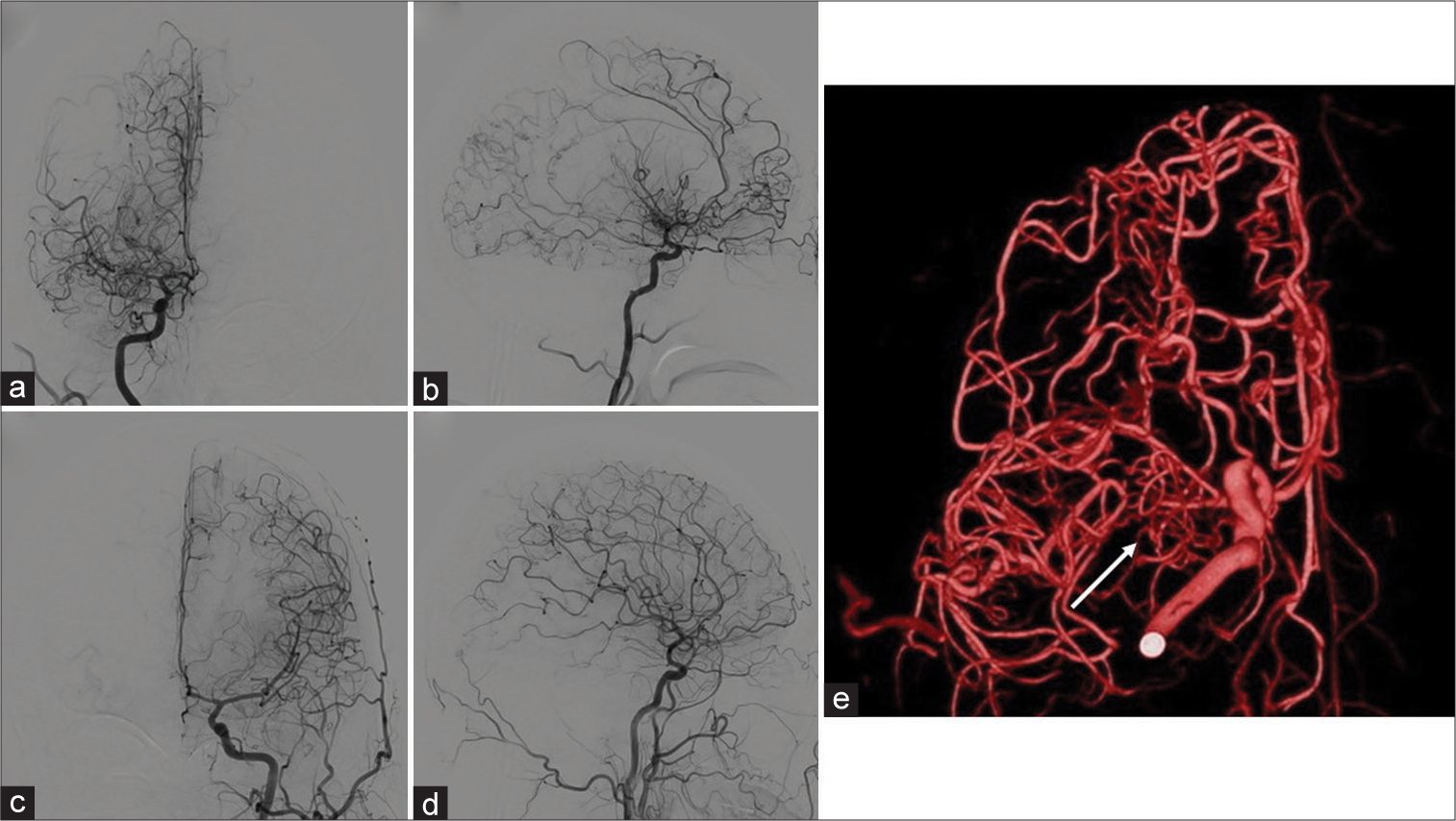
Digital subtraction angiography (DSA) revealed occlusion of the right M1 segment and a plexiform vascular network around the occluded segment [
Figure 5:
Digital subtraction angiograms at the age of 53 show occlusion of the right middle cerebral arteries and the abnormal vascular network around the occluded segment. The vessel diameter of the M2 segment is almost normal. There is no stenosis in the supraclinoid portion of the internal carotid artery (a: Anterior-posterior [A-P] view, b: Lateral view). Left carotid angiogram in normal (c: A-P view, d: Lateral view). (e) Rotational angiograms clearly show a plexiform network formation replacing the M1 occlusion (arrow).
Several laboratory tests were performed to distinguish the etiology. Repetitive blood tests showed that blood counts, thyroid, and renal function were within normal ranges and various items associated with collagen-related disease were negative. In addition, there were no coagulation abnormalities (prothrombin time: 11.2 s, activated partial thromboplastin time: 28.6 s, and D-dimer: 0.5 μg/dL) and cerebrospinal fluid analysis was normal (cells: 1/μL, protein: 37 mg/dL, glucose: 67 mg/dL, and oligoclonal bands: Negative). The antinuclear antibody (ANA) test was positive and showed high antibody titers (ANA test was positive at 1:640, speckled and nucleolar staining patterns were positive at 1:640, and the homo pattern was positive at 1:80). However, test for all other disease-specific antibodies, such as antibodies to double-stranded deoxyribonucleic acid, ribonucleoprotein, Sm, SS-A/Ro, SS-B/La, Scl-70, Jo-1, ribonucleic acid polymerase III, myeloperoxidase anti-neutrophil cytoplasmic antibody (ANCA), and proteinase3-ANCA, was all negative. Although a definitive diagnosis was not reached, the immunological data strongly suggested autoimmune disease involvement.
Bypass surgery was considered due to progression on angiographic findings and hemodynamic deterioration. However, since the patient had been asymptomatic to this point and had without cerebral infarction, conservative follow-up was chosen with strict management of risk factors for atherosclerosis, administration of an antiplatelet drug, and periodic MRI. The patient has remained asymptomatic ever since.
DISCUSSION
T-MCA is a rare vascular anomaly characterized by a replacement of the M1 segment of the MCA by a webbed network of small vessels.[
The etiology of T-MCA is not well understood but it is generally believed that T-MCA is a congenital abnormality resulting from anomalous MCA embryonic development.[
The previous reports have shown that approximately 70% of patients with T-MCA develop hemorrhagic stroke whereas 20% develop ischemic stroke.[
Since the observed ANA staining pattern is not unique to healthy individuals, it was speculated that some immune mechanism was causative.[
In this case, bypass surgery was initially planned because of the progression of angiographic findings and hemodynamic deterioration. However, conservative treatment was chosen since the patient had been asymptomatic for more than 10 years since the initial diagnosis. We previously repeated the efficacy of bypass surgery for T-MCA.[
CONCLUSION
We reported the first case report describing possible de novo T-MCA formation during long-term follow-up. This case demonstrates that T-MCA is not an embryological persistence but may develop secondarily in an acquired manner. Further case accumulation and diagnostic efforts are needed to understand the natural history of T-MCA.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Akkan K, Ucar M, Kilic K, Celtikci E, Ilgit E, Onal B. Unfused or twig-like middle cerebral artery. Eur J Radiol. 2015. 84: 2013-8
2. Brinjikji W, Lanzino G, editors. Letter to the Editor. Rete middle cerebral artery anomalies. J Neurosurg. 2019. 131: 653-4
3. Cekirge HS, Peynircioglu B, Saatci I. Endovascular treatment of an “anterior cerebral artery” aneurysm in a patient with “embryonic unfused middle cerebral artery” anomaly: A case report. Neuroradiology. 2005. 47: 690-4
4. Cho KC, Kim JJ, Jang CK, Hong CK, Joo JY, Kim YB. Rete middle cerebral artery anomalies: A unifying name, case series, and literature review. J Neurosurg. 2018. 131: 453-61
5. Fukuyama R, Yamamura K, Murata H, Miyatake S, Matsumoto N, Abe H. Ruptured aneurysm of and an aplastic or twig-like middle cerebral artery with ring finger protein 213 mutation: A case report. No Shinkei Geka. 2020. 48: 533-40
6. Gailloud P, Albayram S, Fasel JH, Beauchamp NJ, Murphy KJ. Angiographic and embryologic considerations in five cases of middle cerebral artery fenestration. AJNR Am J Neuroradiol. 2002. 23: 585-7
7. Goto Y, Oka H, Hiraizumi S, Okamoto T, Nishii S, Yamamoto H. Aplastic or twig-like middle cerebral artery presenting with intracerebral hemorrhage during pregnancy: Report of two cases. World Neurosurg X. 2019. 2: 100018
8. Goto Y, Nanto M, Oka H, Murakami N, Nakagawa T, Kimura S. Radiological and clinical features of twig-like middle cerebral artery in comparison with moyamoya angiopathy: A multicenter retrospective study. J Neurosurg. 2022. 137: 1718-26
9. Inoue A, Kohno K, Fukumoto S, Ichikawa H, Onoue S, Miyazaki H. A case of ECA-MCA double anastomoses for hemorrhagic type of twig-like MCA. No Shinkei Geka. 2016. 44: 463-71
10. Inoue H, Oomura M, Nishikawa Y, Mase M, Matsukawa N. Aplastic or twig-like middle cerebral artery and cardiogenic cerebral embolism mimicking moyamoya disease with RNF213 polymorphism: A case report. Interv Neuroradiol. 2022. 28: 634-8
11. Kuroda S. Asymptomatic moyamoya disease: Literature review and ongoing AMORE study. Neurol Med Chir (Tokyo). 2015. 55: 194-8
12. Kuroda S, Fujimura M, Takahashi J, Kataoka H, Ogasawara K, Iwama T. Diagnostic criteria for moyamoya disease-2021 revised version. Neurol Med Chir (Tokyo). 2022. 62: 307-12
13. Liu HM, Lai DM, Tu YK, Wang YH. Aneurysms in twig-like middle cerebral artery. Cerebrovasc Dis. 2005. 20: 1-5
14. Lutz T, Mönnings P, Ayzenberg I, Lukas C. Twig-like middle cerebral artery: A seldom vessel anomaly of important relevance. Clin Neuroradiol. 2018. 28: 441-3
15. Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the antinuclear antibody-HEp-2 test is a critical parameter for discriminating antinuclear antibody-positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis Rheum. 2011. 63: 191-200
16. Matsunaga Y, Tsutsumi K, Morofuji Y, Fujimoto T, Kawahara I, Takahata H. A pediatric case of aplastic or twig-like middle cerebral artery presenting with intracerebral hemorrhage. Surg Cereb Stroke. 2014. 42: 51-57
17. Murai Y, Ishisaka E, Watanabe A, Sekine T, Shirokane K, Matano F. Ring finger protein 213 c.14576G>A mutation is not involved in internal carotid artery and middle cerebral artery dysplasia. Sci Rep. 2021. 11: 22163
18. Onoue K, Nguyen TN, Mian A, Dasenbrock H, Bedi H, Abdalkader M. Twig-like middle cerebral arteries: Clinical and radiological findings. Clin Imaging. 2021. 73: 31-7
19. Ota T, Komiyama M. Twig-like middle cerebral artery: Embryological persistence or secondary consequences?. Interv Neuroradiol. 2021. 27: 584-7
20. Ota T, editors. Letter to the Editor. Twig-like middle cerebral artery and moyamoya disease. J Neurosurg. 2022. 137: 1875-6
21. Park J, Hwang JH, Hamm IS. Aneurysm rupture at an anomalous collateral artery that extended from the proximal A2 segment to the middle of the M1 segment, bypassing atresia of the internal carotid artery bifurcation. Case report. J Neurosurg. 2004. 100: 332-4
22. Seki Y, Fujita M, Mizutani N, Kimura M, Suzuki Y. Spontaneous middle cerebral artery occlusion leading to moyamoya phenomenon and aneurysm formation on collateral arteries. Surg Neurol. 2001. 55: 58-62 discussion 62
23. Takarada A, Yanaka K, Onuma K, Nakamura K, Takahashi N, Ishikawa E. Aplastic or twig-like middle cerebral artery harboring unruptured cerebral aneurysms treated by clipping and bypass surgery: Illustrative case. J Neurosurg Case Lessons. 2021. 2: CASE21360
24. Takeda H, Yanaka K, Onuma K, Nakamura K, Ishii K, Ishikawa E. Aplastic or twiglike middle cerebral artery with contralateral middle cerebral artery stenosis showing transient ischemic attack: illustrative case. J Neurosurg Case Lessons. 2022. 3: CASE22121
25. Uchiyama N. Anomalies of the middle cerebral artery. Neurol Med Chir (Tokyo). 2017. 57: 261-6
26. Watanabe A, Kodera M, Sugiura K, Usuda T, Tan EM, Takasaki Y. Anti-DFS70 antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004. 50: 892-900
27. Yamada D, Ishibashi R, Kinosada M, Kurosaki Y, Handa A, Chin M. Aplastic or twig-like cerebral artery with short-term ischemia and bleeding. Jpn J Stroke. 2020. 42: 190-5