- Department of Neurology and Neurosurgery, Federal University of Sao Paulo, São Paulo, Brazil.
- Department of Radiology, Albert Einstein Hospital, São Paulo, Brazil.
- Academic Research Organization, Hospital Albert Einstein, São Paulo, Brazil.
Correspondence Address:
Thiago Pereira Rodrigues, Department of Neurology and Neurosurgery, Federal University of Sao Paulo, São Paulo, Brazil.
DOI:10.25259/SNI_895_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Thiago Pereira Rodrigues1, Mariana Athaniel Silva Rodrigues2, Leonardo Favi Bocca1, Feres Eduardo Chaddad-Neto1, Sergio Cavalheiro1, Edson Amaro Junior2, Gisele Sampaio Silva3, Italo Capraro Suriano1, Ricardo Silva Centeno1. Decompressive craniectomy index: Does the size of decompressive craniectomy matter in malignant middle cerebral artery infarction?. 16-Dec-2022;13:580
How to cite this URL: Thiago Pereira Rodrigues1, Mariana Athaniel Silva Rodrigues2, Leonardo Favi Bocca1, Feres Eduardo Chaddad-Neto1, Sergio Cavalheiro1, Edson Amaro Junior2, Gisele Sampaio Silva3, Italo Capraro Suriano1, Ricardo Silva Centeno1. Decompressive craniectomy index: Does the size of decompressive craniectomy matter in malignant middle cerebral artery infarction?. 16-Dec-2022;13:580. Available from: https://surgicalneurologyint.com/surgicalint-articles/12061/
Abstract
Background: Malignant middle cerebral artery (MCA) infarction is associated with high mortality, mainly due to intracranial hypertension. This malignant course develops when two-thirds or more of MCA territory is infarcted. Randomized clinical trials demonstrated that in patients with malignant MCA infarction, decompressive craniectomy (DC) is associated with better prognosis. In these patients, some prognostic predictors are already known, including age and time between stroke and DC. The size of bone flap was not associated with long-term prognosis in the previous studies. Therefore, this paper aims to further expand the analysis of the bone removal toward a more precise quantification and verify the prognosis implication of the bone flap area/whole supratentorial hemicranium relation in patients treated with DC for malignant middle cerebral infarcts.
Methods: This study included 45 patients operated between 2015 and 2020. All patients had been diagnosed with a malignant MCA infarction and were submitted to DC to treat the ischemic event. The primary endpoint was dichotomized modified Rankin scale (mRS) 1 year after surgery (mRS≤4 or mRS>4).
Results: Patients with bad prognosis (mRS 5–6) were on average: older and with a smaller decompressive craniectomy index (DCI). In multivariate analysis, with adjustments for “age“ and “time” from symptoms onset to DC, the association between DCI and prognosis remained.
Conclusion: In our series, the relation between bone flap size and theoretical maximum supratentorial hemicranium area (DCI) in patients with malignant MCA infarction was associated with prognosis. Further studies are necessary to confirm these findings.
Keywords: Cerebral volumetry, Decompressive craniectomy, Intracranial pressure, Malignant middle cerebral artery infarction
INTRODUCTION
Malignant middle cerebral artery (MCA) infarction is a neurological condition associated with high morbidity and mortality, mainly due to intracranial hypertension secondary to cerebral edema.[
Prognostic predictors in patients who underwent DC after malignant MCA infarction have been explored by several authors since the DECIMAL trial, the first multicenter RCT published in 2007.[
Previous series of patients with malignant MCA infarction that underwent DC analyzed the size of bone flap. Olnhausen et al.[
Studies in patients with traumatic brain injury (TBI) showed that large craniectomy bone flap was associated with better clinical outcome[
Importantly, the method to measure the DC size has been mainly based on bone flap diameter.[
Therefore, this paper aims to further expand the analysis of the bone removal toward a more precise quantification and verify the prognosis implication of the bone flap area/whole supratentorial hemicranium relation in patients treated with DC for malignant middle cerebral infarcts.
MATERIALS AND METHODS
Patients’ selection
This retrospective study was approved by the Institutional Review Board and was performed following the Declaration of Helsinki. All subjects enrolled into this study gave signed consent (in case of decreased level of consciousness, patient’s relatives signed informed consent), and all data were anonymized at source. We enrolled consecutive patients from a single tertiary hospital between 2015 and 2020, with primary admission diagnosis of malignant MCA infarction and age ≥18 years at admission, and requiring DC as clinical treatment for the primary condition.
The diagnosis of malignant MCA infarction was made through a comprehensive analysis of multiple factors, including initial CT scan ischemic areas, National Institutes of Health Stroke Scale (NIHSS), tomographic signs of mass effect, age, and past medical history. Then, a multidisciplinary team including a neurologist assistant, a neurosurgery assistant, a neuroradiologist, and a critical care assistant, made the final decision about whether it has the potential to develop a malignant course or not. In patients that malignant courses were expected, DC was performed as soon as possible. In this study, malignant MCA infarction diagnosis included patients with a large infarction in vascular territory of MCA including or not another vascular territory (anterior cerebral artery or posterior cerebral artery).
Clinical and radiological data were collected from the electronic medical records and image server, and included age, sex, laterality of infarction, time from onset of symptoms until DC, NIHSS score, vascular territory’s area, medical history, alteplase use, brain endovascular procedures before DC, midline shift (MLS) peak time, and modified Rankin scale (mRS) after 1 year.
Surgical procedure
All procedures were performed by board certified neurosurgeons from our hospital neurosurgical department. Two types of skin flaps were made, according to surgeon’s personal preference: a large inverted question mark or “T” type skin flap (Kempe’s incision[
Image analysis
All CT examinations performed during patients’ clinical care were analyzed and processed in the software 3D Slicer (v. 4.10 –
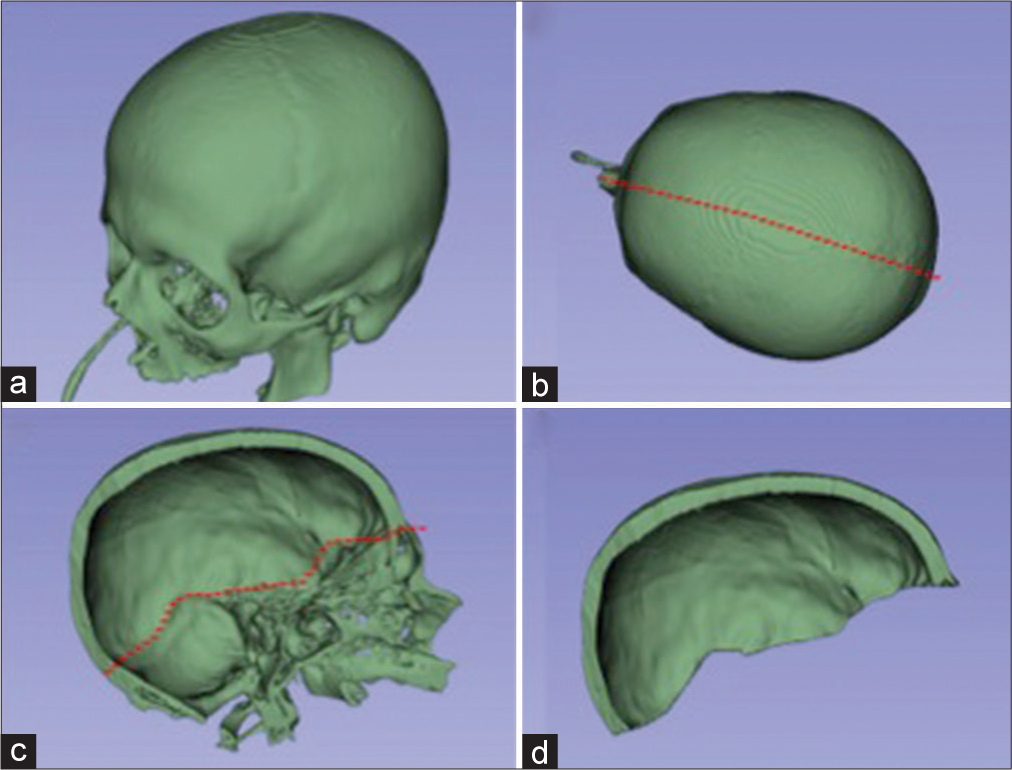
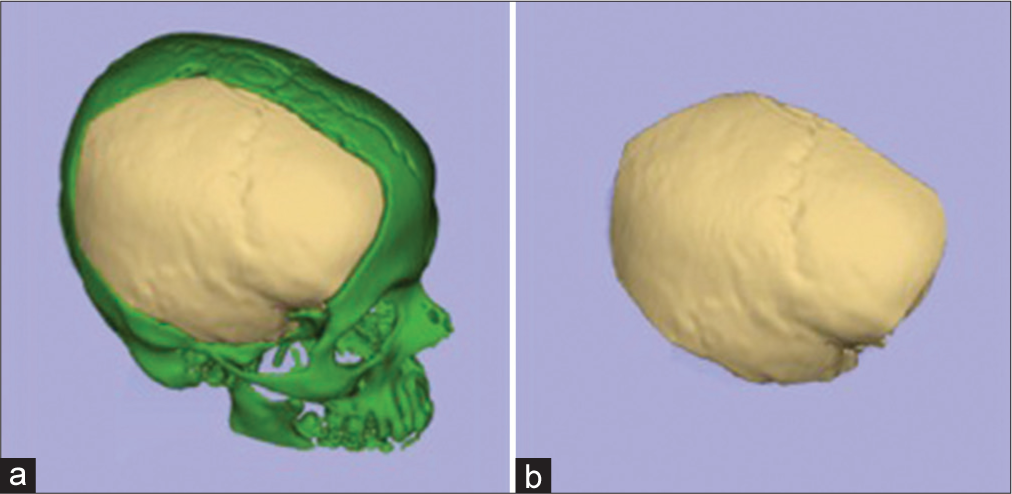
Manual bone segmentation of all supratentorial hemicranium was performed using the sagittal suture, internal occipital protuberance, groove for transverse sinus, the intersection between the middle fossa floor and the lateral wall, and the intersection between anterior cranial fossa floor and lateral/anterior wall as the borders. This area was considered maximum theoretical supratentorial hemicranium bone flap [
Figure 1:
Bone segmentation of preoperative C.T. examination. (a) All cranial bone segmented. (b) The red-dotted line represents a cut passing through external occipital protuberance, sagittal suture, and internasal suture. (c) A left-sided hemicranium with the red-dotted line representing a cut that passes through internal occipital protuberance, groove for transverse sinus, intersection between the middle fossa floor and the lateral wall, and the intersection between anterior cranial fossa floor and lateral/anterior wall. (d) The maximum theoretical hemispheric hemicranium bone flap.
Transverse CT in aligned to orbitomeatal line was used for the measurement of maximum craniectomy diameter.
Statistical analysis
The categorical variables were described as numbers of cases and percentages, and the quantitative variables were characterized as means ± standard deviations.
For comparison between groups (favorable or unfavorable), continuous variables were compared with Mann–Whitney U-test, while categorical variables were analyzed by Fisher’s exact test.
A multivariate logistic regression model was performed, using the “enter” method, to identify if DCI was independently associated with worse outcome, with adjustment for age and time from symptoms onset to DC.
The correlation between maximum craniectomy diameter and DCI, and between age and DCI was tested using Pearson’s correlation test. The interaction between significant variable in the regression model was checked using Wald test.
For the statistically significant association, P < 0.05 was used (for multivariate model inclusion, we use P < 0.1). All analyses were performed using the statistical program IBM Statistical Package for the Social Sciences version 26.
RESULTS
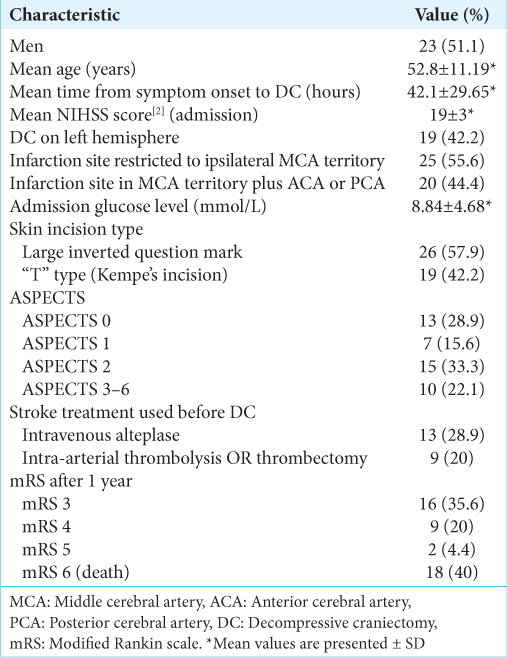
We included 45 patients who underwent a DC after the diagnosis of a malignant MCA infarction in our hospital from 2015 to 2020. The mean age at DC was 52.8 years. The mean time between the ischemic ictus and DC was 42.1 h. Isolated MCA infarction occurred in 25 patients; MCA plus ACA occurred in nine patients; MCA plus ACP occurred in six patients; and MCA plus ACA plus ACP occurred in five patients. Regarding skin flap, large inverted question mark was more frequent than “T” type (26 vs. 19 patients). All patients had NIHSS scores higher than 13 [
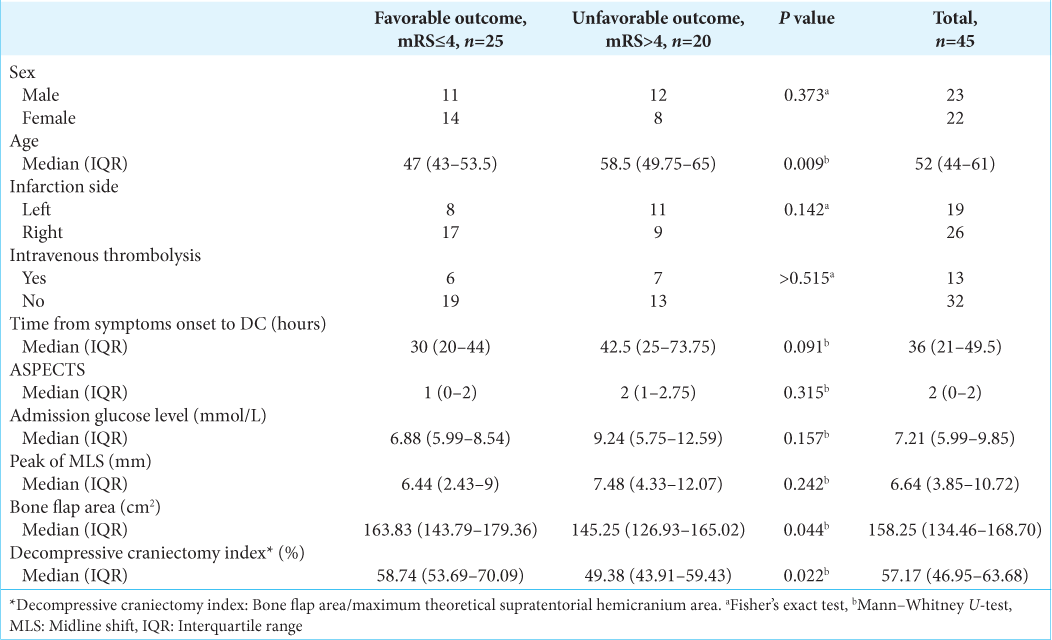
In comparison between groups, patients with bad prognosis (mRS 5–6) were on average: older (P = 0.009) and with a smaller DCI (P = 0.022). Other factors did not significantly differ between favorable (n = 25) versus unfavorable (n = 20) group, which included sex, infarction side, intravenous thrombolysis using time from symptoms onset to DC (P = 0.091), ASPECTS score, admission glucose level, and peak of MLS [
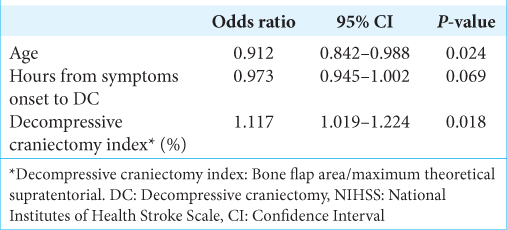
In the multivariate model, we included three factors that met the criteria (P < 0.1 in univariate analysis): age (P = 0.009), time from symptoms onset to DC (P = 0.091), and DCI (P = 0.022). Bone flap area was excluded from the multivariate model because of multicollinearity issues [
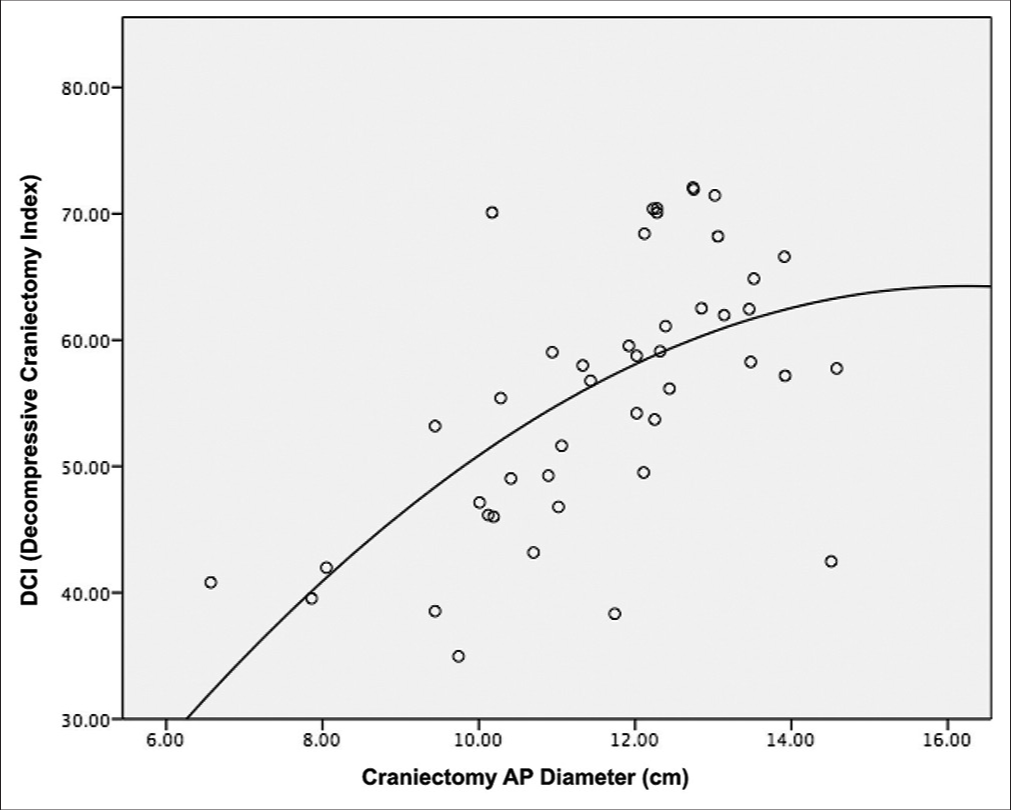
The Pearson’s correlation between DCI and maximum craniectomy diameter was 0.594 (P < 0.001) and the scatter plot is shown in
The interaction between age and DCI in regression model was ruled out (Wald test = 0.609, P = 0.435).
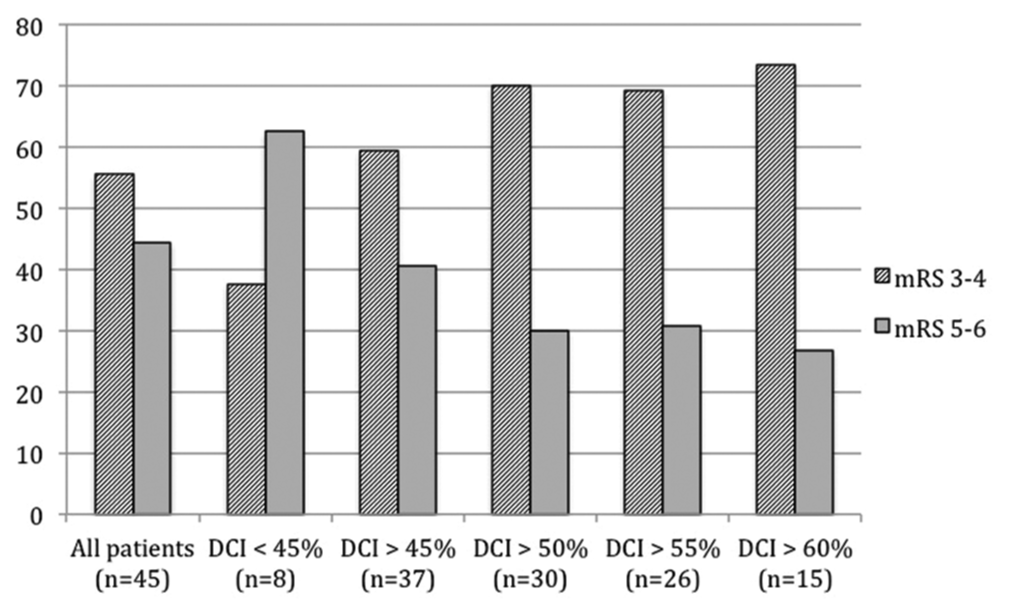
The analysis of dichotomized outcomes according to DCI cutoffs is shown in
DISCUSSION
In this study, we evaluated all patients who underwent a DC after a malignant MCA infarction in a single center from 2015 to 2020. In this series, we identified three possible predictors: age, time from symptoms onset to DC (P = 0.069), and the relation between bone flap area and maximum theoretical supratentorial hemicranium area DCI.
Age as a predictive factor in patients with malignant MCA infarction that underwent DC was already demonstrated by several authors. In Carter et al.[
Time from symptoms onset to DC has already identified as a predictive factor by the previous studies. Schwab et al.,[
Craniectomy bone flap size was also previously evaluated in literature. von Olnhausen et al.[
In this series, for the purpose of analyzing bone flap area, we employed an open-source graphic software and, thus, we measured the area of the bone flap. This measurement took into account the curvature and the shape. Furthermore, we also considered the bone area of supratentorial hemicranium. The high relation between bone flap area and maximum theoretical supratentorial hemicranium area, that is, DCI, was significantly associated with good prognosis. Despite the fact that high DCI was significantly associated with better prognosis, the odds ratio was low: 1.117, therefore, the magnitude of the effect was low. We think that other unevaluated factors could also be predictive factors.
In our opinion, most of neurosurgeons agree that size matters in DC and the minimal craniectomy diameter established by Wagner et al.[
In our series, we had a low frequency of therapeutic thrombolysis (28.9% of intravenous alteplase use and 20% of intra-arterial or mechanical thrombectomy). It happened mainly because of two factors: delayed time between initial ictus and arrival at hospital, and the fact that most of our patients arrived at our hospital coming from other hospitals that do not have stroke team and, therefore, are not able to implement thrombolysis therapies.
This retrospective study has several limitations. First, it is based on a small sample size in a single institution. Second, because of the limited number in this series, we could not define a precise cutoff for DCI.
Apart from the discussion about whether to perform or not the DC in patients with malignant MCA infarction (that in our view is well-established in literature), we think that technical aspects emphasizing how to perform a DC in patients with malignant MCA infarction should be better evaluated.
Concisely, in this study, we described a method to calculate craniectomy bone flap area. Considering the maximum theoretical supratentorial hemicranium area and the craniectomy bone flap area, we obtained the DCI. Then, in a 45 patients’ series of malignant MCA infarction that underwent DC, we found three prognostic predictors: age, hours from symptoms onset to DC, and DCI.
CONCLUSION
In our series, the relation between bone flap size and theoretical maximum supratentorial hemicranium area DCI in patients with malignant MCA infarction was associated with prognosis. Further studies are necessary to confirm these findings.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, editors. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017. 80: 6-15
2. Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997. 40: 1168-75
3. Das S, Mitchell P, Ross N, Whitfield PC. Decompressive hemicraniectomy in the treatment of malignant middle cerebral artery infarction: A meta-analysis. World Neurosurg. 2019. 123: 8-16
4. Daou B, Kent AP, Montano M, Chalouhi N, Starke RM, Tjoumakaris S. Decompressive hemicraniectomy: Predictors of functional outcome in patients with ischemic stroke. J Neurosurg. 2016. 124: 1773-9
5. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. 2012. 30: 1323-41
6. Hinduja A, Samant R, Feng D, Hannawi Y. Herniation despite decompressive hemicraniectomy in large hemispherical ischemic strokes. J Stroke Cerebrovasc Dis. 2018. 27: 418-24
7. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009. 8: 326-33
8. Holtkamp M, Buchheim K, Unterberg A, Hoffmann O, Schielke E, Weber JR. Hemicraniectomy in elderly patients with space occupying media infarction: improved survival but poor functional outcome. J Neurol Neurosurg Psychiatry. 2001. 70: 226-8
9. Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: A multicenter, prospective, randomized controlled study. J Neurotrauma. 2005. 22: 623-8
10. Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): A randomized, controlled trial. Stroke. 2007. 38: 2518-25
11. Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014. 370: 1091-100
12. Kempe LG, editors. Hemispherectomy. Operative Neurosurgery, Cranial, Cerebral, and Intracranial Vascular Disease. Germany: Springer-Verlag; 1968. 1: 180-90
13. Li G, Wen L, Yang XF, Zheng XJ, Zhan RJ, Liu WG. Efficacy of large decompressive craniectomy in severe traumatic brain injury. Chin J Traumatol. 2008. 11: 253-6
14. Neugebauer H, Fiss I, Pinczolits A, Hecht N, Witsch J, Dengler NF. large size hemicraniectomy reduces early herniation in malignant middle cerebral artery infarction. Cerebrovasc Dis. 2016. 41: 283-90
15. Paliwal P, Kazmi F, Teoh HL, Yeo LL, Seet RC, Yeo TT. Early decompressive hemicraniectomy for malignant middle cerebral artery infarction in Asian Patients: A single-center study. World Neurosurg. 2018. 111: 722-8
16. Schur S, Martel P, Marcoux J. Optimal bone flap size for decompressive craniectomy for refractory increased intracranial pressure in traumatic brain injury: Taking the patient’s head size into account. World Neurosurg. 2020. 137: 430-6
17. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998. 29: 1888-93
18. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007. 6: 215-22
19. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke. 2007. 38: 2506-17
20. Vibbert M, Mayer SA. Early decompressive hemicraniectomy following malignant ischemic stroke: The crucial role of timing. Curr Neurol Neurosci Rep. 2010. 10: 1-3
21. von Olnhausen O, Thorén M, von Vogelsang AC, Svensson M, Schechtmann G. Predictive factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. Acta Neurochir (Wien). 2016. 158: 865-72
22. Wagner S, Schnippering H, Aschoff A, Koziol JA, Schwab S, Steiner T. Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. J Neurosurg. 2001. 94: 693-6