- Department of Neurosciences, University of the Witwatersrand, Johannesburg, Gauteng, South Africa.
DOI:10.25259/SNI_303_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dinesh Naidoo. Decompressive craniotomy for malignant middle cerebral artery infarction: The quest for an African perspective. 03-May-2021;12:200
How to cite this URL: Dinesh Naidoo. Decompressive craniotomy for malignant middle cerebral artery infarction: The quest for an African perspective. 03-May-2021;12:200. Available from: https://surgicalneurologyint.com/surgicalint-articles/10783/
Abstract
Background: Although associated with controversy, decompressive craniotomy (DC) for malignant middle cerebral artery infarction (MMCAI) is an unequivocally lifesaving intervention. DC for MMCAI is rarely performed in lower- to middle-income countries.
Methods: A systemic review was performed in attempt to determine the rates of utilization and outcomes of DC on the African continent.
Results: Only two African studies describing DC for MMCAI were found.
Conclusion: DC for MMCAI is rarely performed and/or reported on the African continent. The African perspective for this needs to be urgently broadened.
Keywords: Resource poo, African perspective, Decompressive craniotomy, Functional outcome, Malignant middle cerebral artery infarction
INTRODUCTION
Malignant middle cerebral artery (MCA) infarction (MMCAI) is a condition characterized by rapid neurological deterioration following a large territory (≥50% of involved hemisphere) infarction of the MCA.[
Decompressive craniotomy (DC) is a procedure in which a large portion (typically more than 12 cm) of the skull is removed and the dura is opened,[
The finding of increased survival at the expense of moderate-to-severe disability[
The foregoing notwithstanding, the findings of increased survival rates of patients with moderate-to-severe disability (mRSe ≥ 3) continue to raise important ethical considerations,[
We present a 4-case series of MMCAI with and without DC, all unique with respect to each other, and subsequently sought to determine the rate of utilization of DC for MMACI, and the outcomes thereof, on the African continent. Stated differently, an African perspective for MMCAI was sought at a time when the burden of general stroke care apears to be increasing on the African continient.[
CASE 1
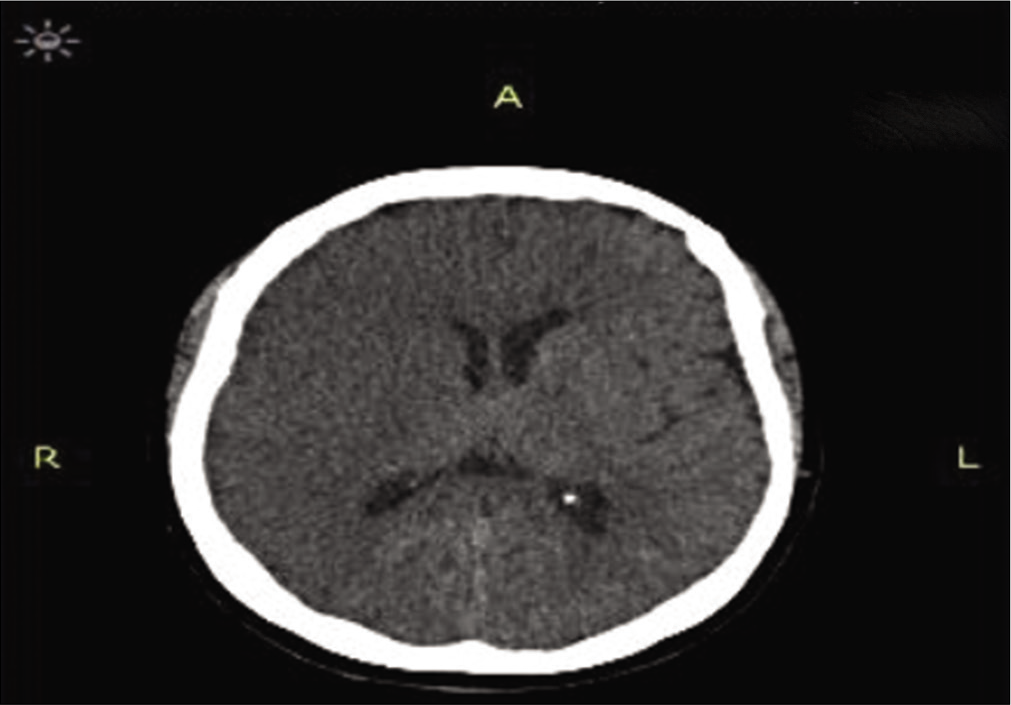
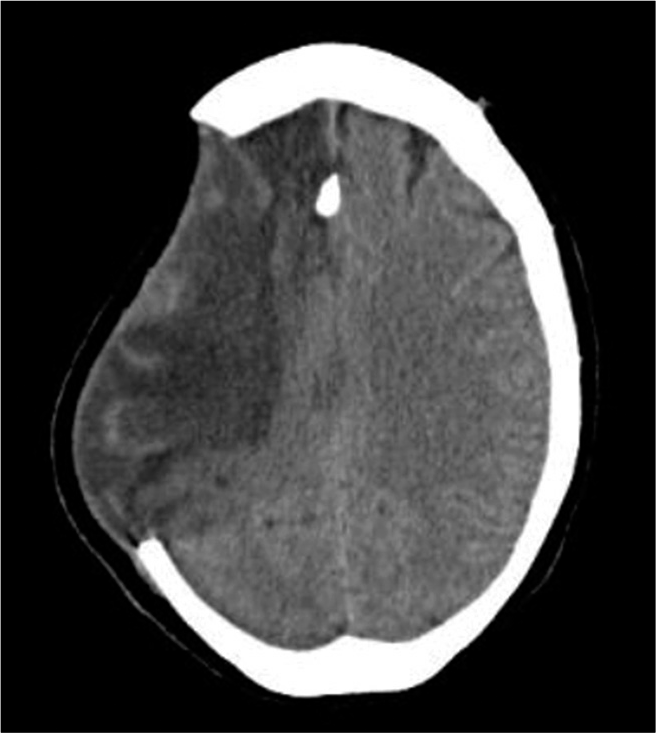
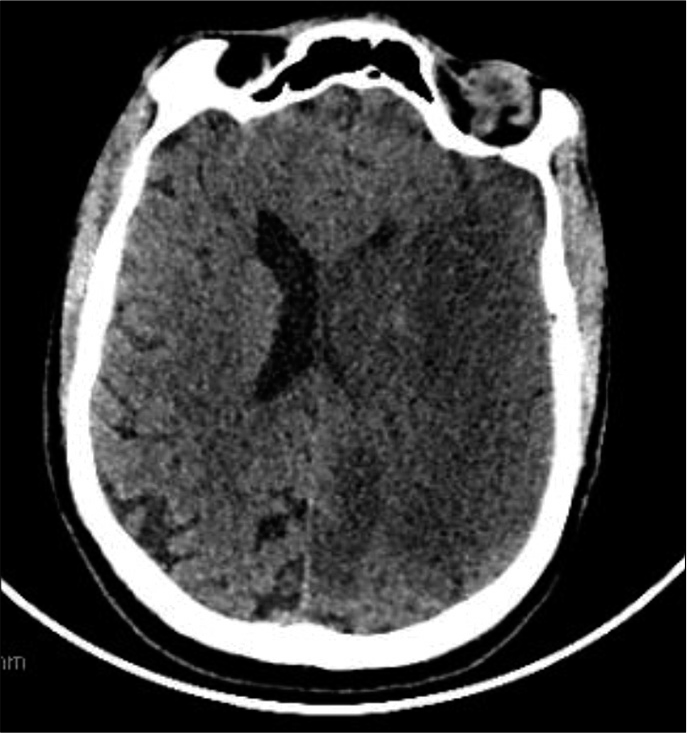
This obtained from next of kin presented with a sudden onset of dense left-sided weakness. CT scan approximately 12 h after symptom onset showed a dense right MCA infarction but with minimal mass effect and midline shift [
CASE 2
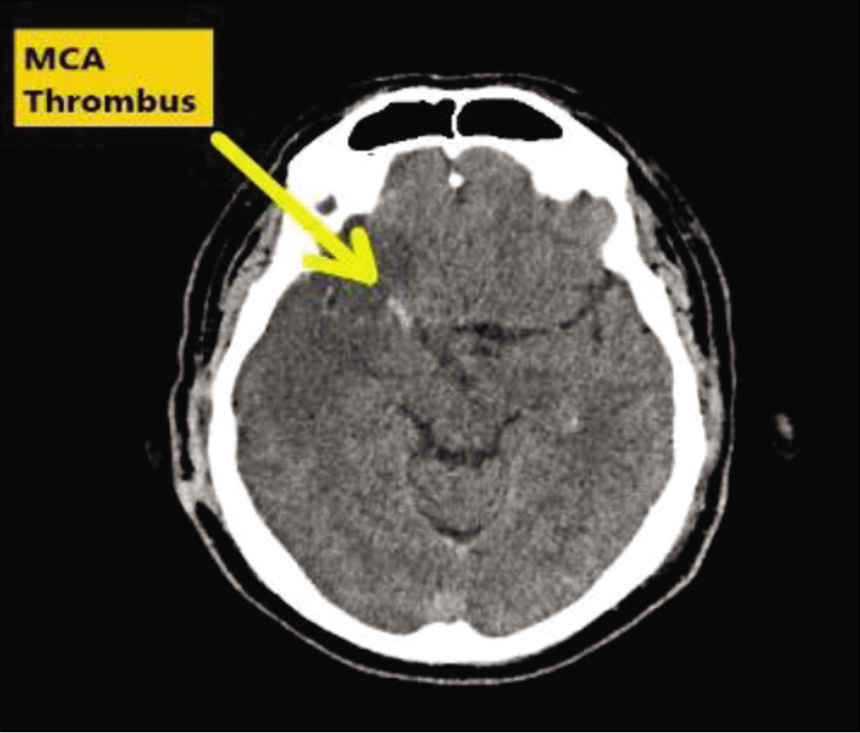
A 48-year-old female presented to hospital within 6 h of a sudden onset of left-sided weakness. Fully conscious on arrival the patient was given intravenous thrombolytic therapy after an initial CT scan demonstrated no areas of established infarction and no areas of hemorrhage [
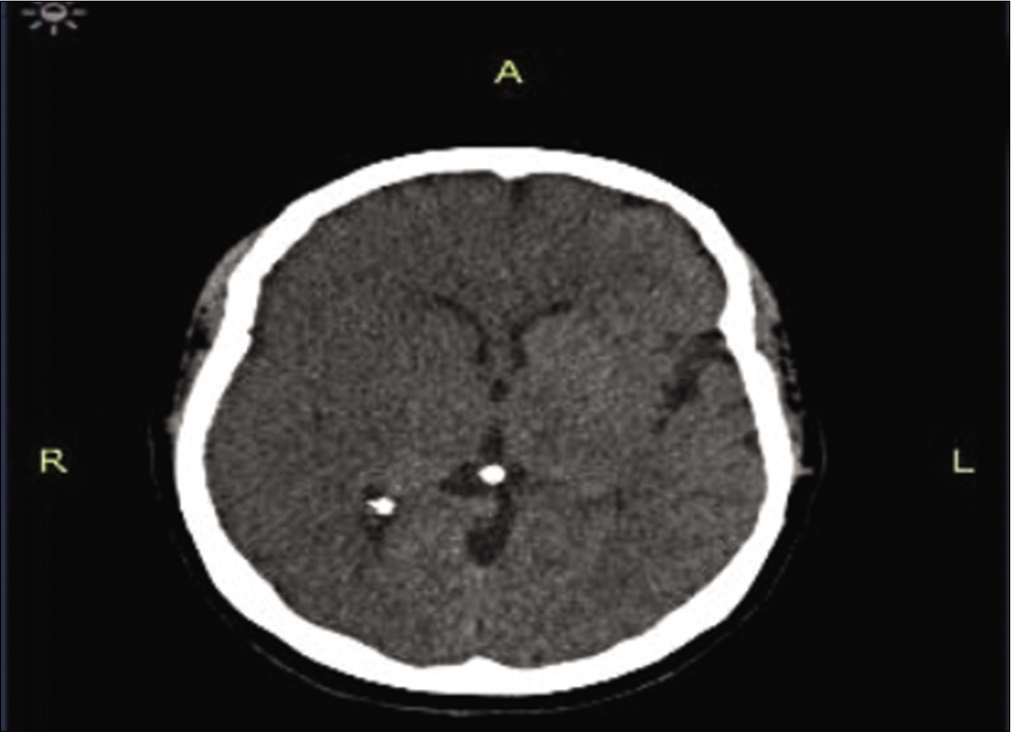
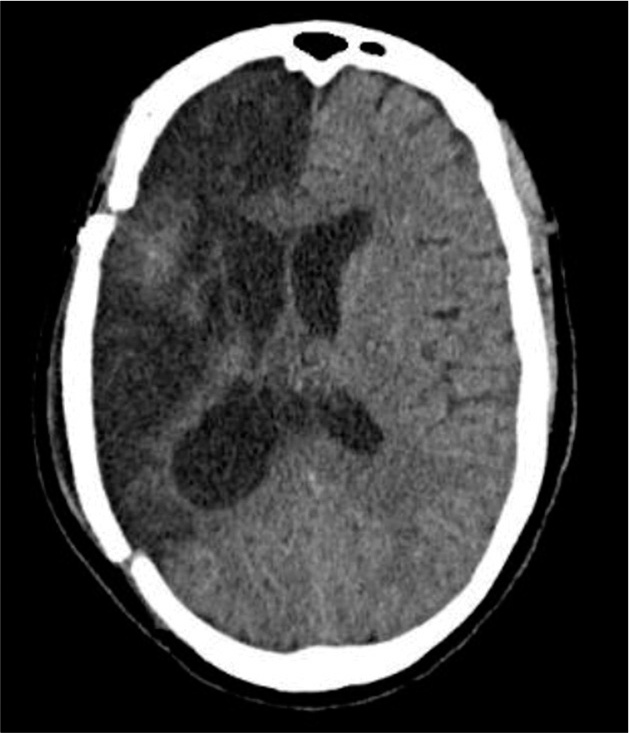
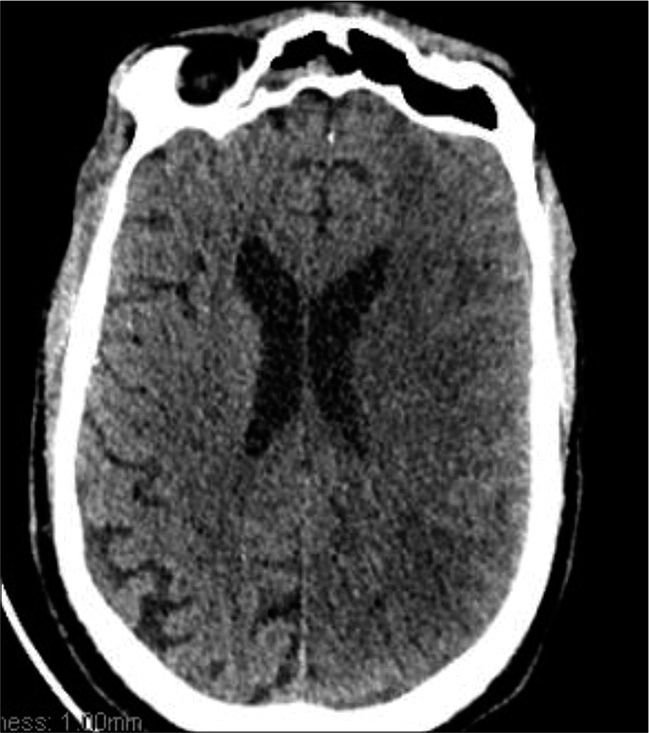
The patient gradually becomes more confused and an MRI scan was done ~20 h later. This MRA demonstrated a massive right side MCA infarction with early midline shift and ventricular effacement [
Once the brain swelling had settled [
CASE 3
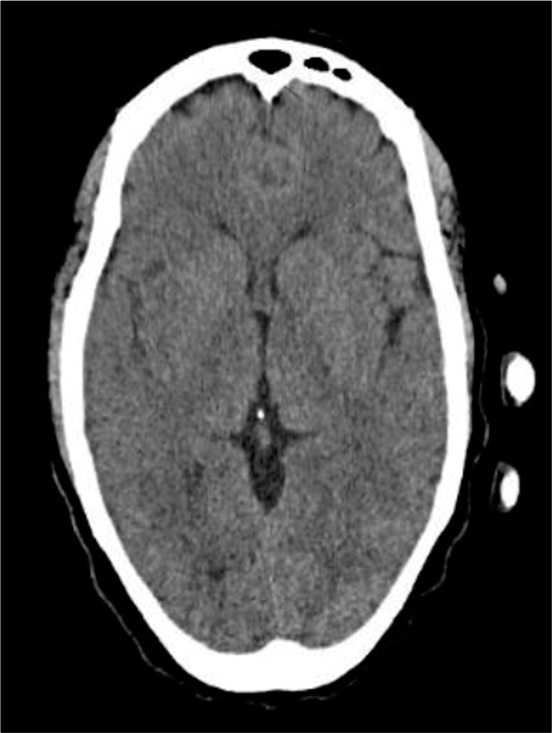
A 53-year-old man with multiple cardiac comorbidities was admitted to the hospital with left-sided weakness sided weakness and obtundation. CT scan demonstrated a massive right sided MCA infarction [
CASE 4
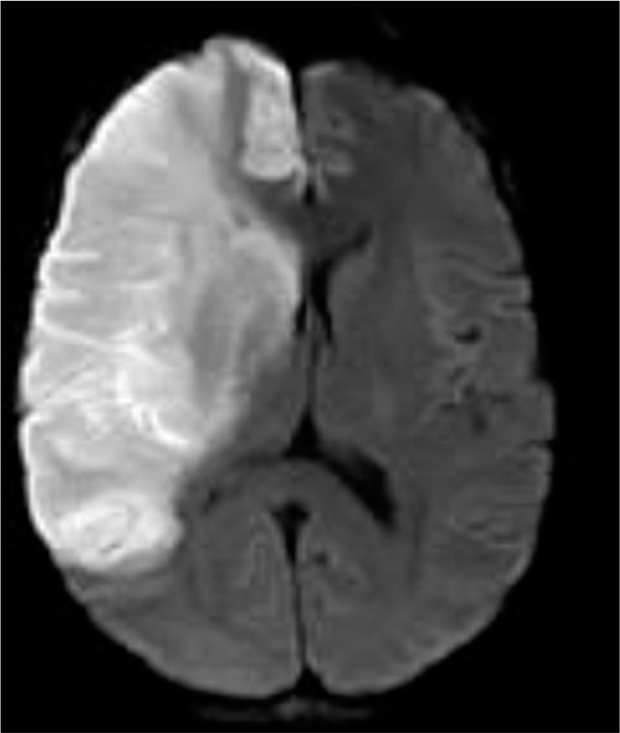
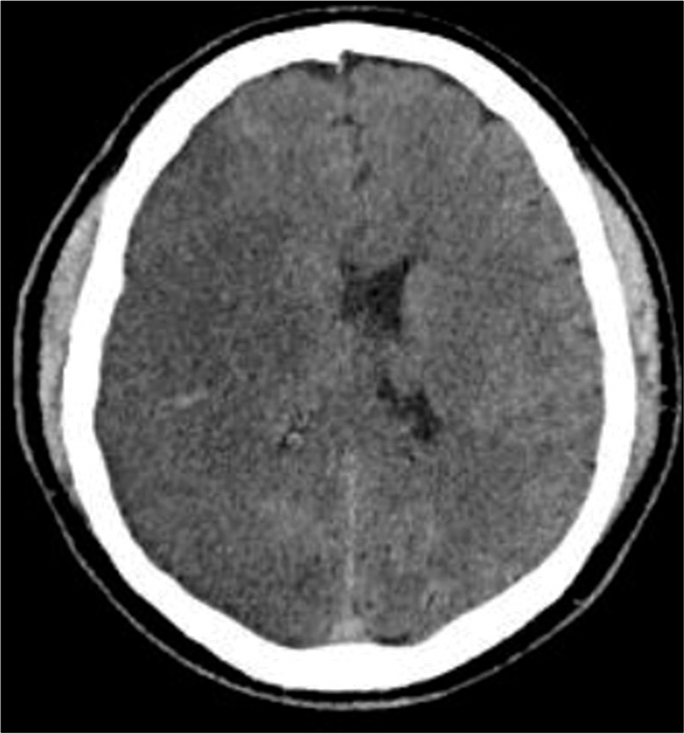
A 41-year-old male presented with sudden onset right-sided hemiplegia and aphasia, the patients Glasgow Coma Score was 11/11VA. CT scan done the following day showed massive left-sided MCA infarct with loss of sulcation (mass effect) but no midline shift [
SYSTEMIC REVIEW
Objective
Using the PICOS framework, the objectives of the review were as follows;
Patients, the identification of studies involving patients on the African continent with malignant MCA syndrome.
Interventions, patients on the African continent with MMCAI who had undergone DC.
C, the comparator to the intervention would be nonoperative treatment of malignant MCA infarction, synonyms for nonoperative treatment include medical or conservative treatment.
Outcomes, we intended to describe both patient survival rates and neurological outcomes, using the mRS for the latter.
Study design, study designs were not specified particularly because there was intended to be qualitative in nature and because an initial scouting review suggested a paucity of studies.
The review was conceptualized in accordance with the Preferred Reporting Items for Meta-Analysis and Systemic Reviews principles. The strategy involved a search of Medline (via PubMed) and Web of Science and African Journals Online (AJOL) databases.
The initial abstract search was done using the terms malignant MCA (stroke OR infraction OR syndrome) AND decompressive (craniectomy OR craniotomy OR hemicraniectomy).
The search was limited to scholarly literature published in the English language. All article types were considered for inclusion including editorials, opinion pieces, and letters to editors.
No outcome measures were specified for the abstract search.
Inclusion criteria
The following criteria were included in the study:
Patients with MMCAI Adult patients Patients with massive MCA infarction who underwent decompressive cranial surgery at any stage after the diagnosis.
Exclusion criteria
The following criteria were excluded from the study:
Studies not, wholly or partially, carried out on the African content Studies confined to pediatric patients Patients with confirmed MMCAI treated medically only DC performed for cerebellar stroke or condition other than MMCAI (such as venous sinus thrombosis) MMCAI patients treated with strokectomy with or without preservation of skull integrity Studies not conducted on human subjects.
Study selection and data extraction
All potential studies, screened at the abstract stage by all authors, that met the inclusion criteria were imported into a reference manager to avoid duplication. A fundamental assumption of the study was that, unless specifically stated otherwise, the author affiliation was reflective of the study setting except for meta-analyses and systemic reviews.
The abstracts of all studies were analyzed for exclusion criteria and where this could be determined the study was discarded without full-text screening. Full-text screening was performed when inclusion and exclusion criteria could not be assessed by abstract screening.
To be included in the final analyses, studies had to meet all the inclusion criteria and not meet any of the exclusion criteria.
RESULTS
The database search yielded the following results:
Web of Science: 483 results (accessed on February 21, 2021) PubMed: 395 results (accessed January 25, 2021) AJOL: 1 result (accessed January 31, 2021).
Only three studies did not meet the exclusion criteria, all originated from Egypt.
The first study was a prospective randomized study comparing delayed DC (Group 1) versus early DC (Group2) for MMCAI. In the first group, patients were only operated on once their conscious levels began to deteriorate. In the second group, surgery was performed within 6 h of MMCAI. Group 1 compromised 27 patients and Group 2 19 patients.[
The second noncontrolled study prospectively analyzed 24 patients who underwent DC for MMCAI and refractory intracranial hypertension over a 3-year period.[
The third study described a novel technique using glue for the fixation of skull flaps following craniotomy.[
The single article from the AJOL search was a Nigerian review article on DC focused largely on the performance of DC for trauma.[
DISCUSSION
Large multicenter controlled cohort studies are unlikely to be undertaken in Africa and indeed even the developed world, given the proven benefit of DC for MMCAI. Instead, case reporting such at the included illustrative cases and the two from Egypt may help guide decision-making for those who find themselves in similar contexts.
The paucity of studies of DC for MMCAI on the African continent may be reflective of the relative lack of regional stroke literature.[
The most likely reason for the paucity of literature describing DC for MMCAI on the African continent, however, is likely the reluctance of clinicians to offer DC to patients with MMCAI because of a pervasive perception that the procedure would lead, inevitably, to dependent and poor neurological outcomes. Das et al. in their meta-analysis of professional and patient views of DC for MMCAI concluded that “professionals think that surgery is not worth the high disability rate.”[
DC for malignant MCA syndrome is an unequivocally lifesaving procedure and an essential component of modern-day stroke care.[
The two Egyptian studies (although neither being controlled),[
CONCLUSION
DC for MMCAI will continue to be performed on an individual,[
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adeoye O, Hornung R, Khatri P, Ringer A, Kleindorfer D. The rate of hemicraniectomy for acute ischemic stroke is increasing in the United States. J Stroke Cerebrovasc Dis. 2011. 20: 251-4
2. Baatiema L, Chan CKY, Sav A, Somerset S. Interventions for acute stroke management in Africa: A systematic review of the evidence. Syst Rev. 2017. 6: 213
3. Back L, Nagaraja V, Kapur A, Eslick GD. Role of decompressive hemicraniectomy in extensive middle cerebral artery strokes: A meta-analysis of randomised trials. Intern Med J. 2015. 45: 711-7
4. Bryer A, Connor M, Haug P, Cheyip B, Staub H, Tipping B. South African guideline for management of ischaemic stroke and transient ischaemic attack 2010: A guideline from the South African stroke society (SASS) and the SASS writing committee. S Afr Med J. 2010. 100: 747-78
5. Daniel CH, du Trevou MD. A survey of selected key areas of management of South African neurosurgical patients. S Afr J Surg. 2017. 55: 55-61
6. Das S, Mitchell P, Ross N, Whitfield PC. Decompressive hemicraniectomy in the treatment of malignant middle cerebral artery infarction: A meta-analysis. World Neurosurg. 2019. 123: 8-16
7. de-Graft Aikins A, Unwin N, Agyemang C, Allotey P, Campbell C, Arhinful D. Tackling Africa’s chronic disease burden: From the local to the global. Global Health. 2010. 6: 5
8. Eghwrudjakpor PO, Allison AB. Decompressive craniectomy following brain injury: Factors important to patient outcome. Libyan J Med. 2010. 5: 4620
9. Elsawaf A, Galhom A. Decompressive craniotomy for malignant middle cerebral artery infarction: Optimal timing and literature review. World Neurosurg. 2018. 116: e71-8
10. Elsayed A, Elsayed A. Decompressive craniectomy in malignant hemispheric infarction: Favorable outcome and disability. Egypt J Neurol Psychiatr Neurosurg. 2019. 55: 25
11. Honeybul S, Ho KM, Gillett G. Outcome following decompressive hemicraniectomy for malignant cerebral infarction ethical considerations. Stroke. 2015. 46: 2695-8
12. Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016. 375: 1119-30
13. Huttner HB, Schwab S. Malignant middle cerebral artery infarction: Clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. 2009. 8: 949-58
14. Li YP, Hou MZ, Lu GY, Ciccone N, Wang XD, Dong L. Neurologic functional outcomes of decompressive hemicraniectomy versus conventional treatment for malignant middle cerebral artery infarction: A systematic review and meta-analysis. World Neurosurg. 2017. 99: 709-25.e3
15. Nkoke C, Luchuo EB. Post-stroke care: An alternative model to reduce stroke related morbidity in Sub-Saharan Africa. Ann Transl Med. 2015. 3: 238
16. Owolabi MO, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C. The burden of stroke in Africa: A glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015. 26: S27-38
17. Rajwani KM, Crocker M, Moynihan B. Decompressive craniectomy for the treatment of malignant middle cerebral artery infarction. Br J Neurosurg. 2017. 31: 401-9
18. Soinne L, Sundararajan S, Strbian D. Malignant hemispheric infarction: Diagnosis and management by hemicraniectomy. Stroke. 2014. 45: 185-7
19. Sultan A, Mohamed A. Efficacy and safety of using N-butyl cyanoacrylate in cranial fixation following trauma and other pathologies. Turk Neurosurg. 2018. 28: 416-20
20. Tanrikulu L, Oez-Tanrikulu A, Weiss C, Scholz T, Schiefer J, Clusmann H. The bigger, the better? About the size of decompressive hemicraniectomies. Clin Neurol Neurosurg. 2015. 135: 15-21
21. Treadwell SD, Thanvi B. Malignant middle cerebral artery (MCA) infarction: Pathophysiology, diagnosis and management. Postgrad Med J. 2010. 86: 235-42
22. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007. 6: 215-22