- Department of Neurosurgery, University of Illinois at Chicago, Chicago, Illinois, USA
Correspondence Address:
Ankit I. Mehta
Department of Neurosurgery, University of Illinois at Chicago, Chicago, Illinois, USA
DOI:10.4103/sni.sni_325_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gregory D. Arnone, Darian R. Esfahani, Steven Papastefan, Neha Rao, Prateek Kumar, Konstantin V. Slavin, Ankit I. Mehta. Diabetes and morbid obesity are associated with higher reoperation rates following microvascular decompression surgery: An ACS-NSQIP analysis. 01-Nov-2017;8:268
How to cite this URL: Gregory D. Arnone, Darian R. Esfahani, Steven Papastefan, Neha Rao, Prateek Kumar, Konstantin V. Slavin, Ankit I. Mehta. Diabetes and morbid obesity are associated with higher reoperation rates following microvascular decompression surgery: An ACS-NSQIP analysis. 01-Nov-2017;8:268. Available from: http://surgicalneurologyint.com/surgicalint-articles/diabetes-and-morbid-obesity-are-associated-with-higher-reoperation-rates-following-microvascular-decompression-surgery-an-acs%e2%80%91nsqip-analysis/
Abstract
Background:Microvascular decompression (MVD) is the preferred treatment for refractory trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia. Despite its high rate of success, MVD carries risk of complications. In this study, we examine outcomes following MVD and identify risk factors associated with adverse outcomes.
Methods:A review of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database was performed with CPT code 61458 queried between 2007 and 2014. Demographics, preoperative comorbidities, and 30-day outcomes were analyzed. Univariate and multivariate regression analyses were performed to identify predictors of reoperation and adverse events.
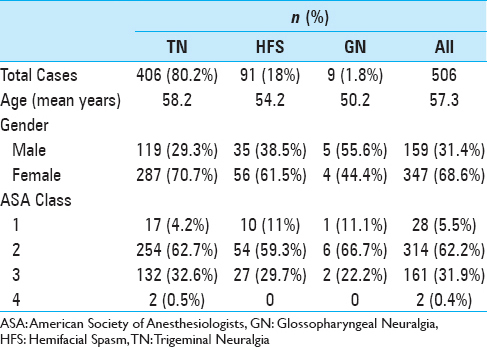
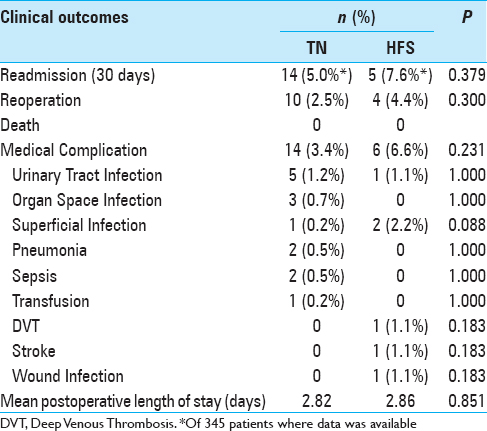
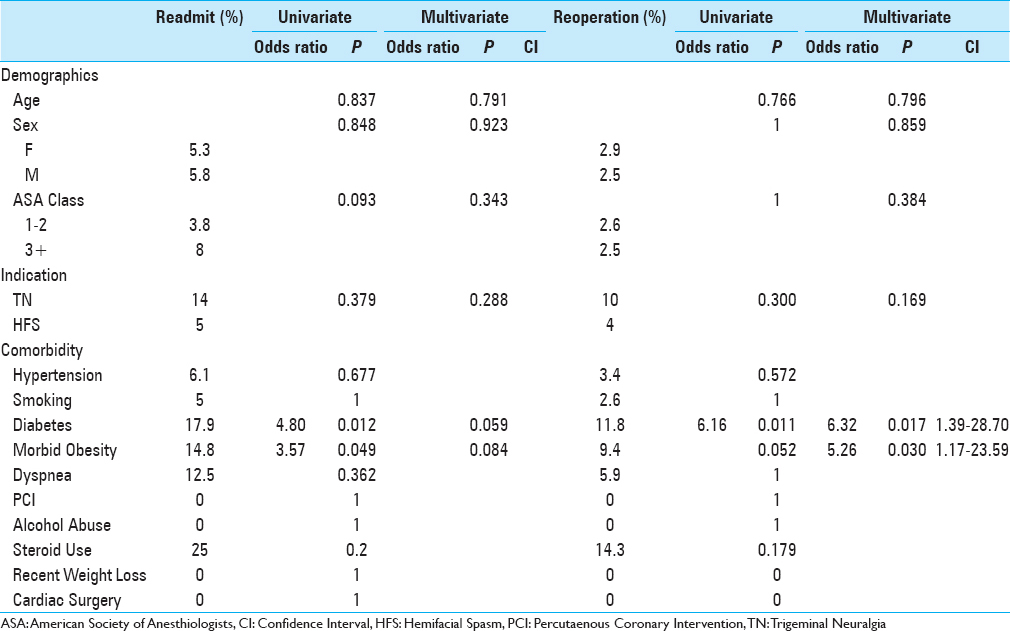
Results:Five hundred and six craniotomies were studied. Nineteen (5.5%) instances of 30-day readmission were reported, with 14 (2.8%) patients returning to the operating room. No instances of death or hemorrhage requiring operation were reported. Morbid obesity (body mass index >40) (P = 0.030) and diabetes (P = 0.017) were associated with risk of reoperation. Age, operative time, and indication for surgery were not associated with significant differences in adverse events.
Conclusions:MVD is a common and effective procedure with a relatively safe profile and low 30-day risk of reoperation. Advanced age is not associated with worse outcomes. Obesity and diabetes, however, are associated with increased risk of reoperation and may warrant additional precautions.
Keywords: Diabetes, microvascular decompression, NSQIP, obesity, readmission, reoperation
INTRODUCTION
Microvascular decompression (MVD) is the preferred surgical treatment for refractory trigeminal neuralgia (TN), hemifacial spasm (HFS), and glossopharyngeal neuralgia (GN), debilitating conditions of the 5th, 7th, and 9th cranial nerves, respectively.[
The frequent use of MVD in TN, HFS, and GN is attributable to a high success rate and durability over time.[
The American College of Surgeons–National Surgical Quality Improvement Program (ACS-NSQIP) collects information from many types of surgeries in a standardized, risk-adjusted database with the aim of providing metrics for patient outcome improvement, and has been has shown to decrease morbidity and mortality in participating hospitals.[
MATERIALS AND METHODS
A retrospective review of the prospectively-collected ACS-NSQIP database was performed. Data from 2007 to 2014 was investigated using primary Current Procedural Terminology (CPT) code 61458 (craniotomy, suboccipital; for exploration or decompression of cranial nerves). Only cases with ICD-9 (International Classification of Diseases) codes 350.1 (trigeminal neuralgia), 351.8 (other facial nerve disorders), 351.9 (facial nerve disorder), and 352.1 (glossopharyngeal neuralgia) were considered. Project approval was obtained through the university institutional review board. As data collection involved no risk to participants and all NSQIP data is anonymized, a waiver for consent was granted.
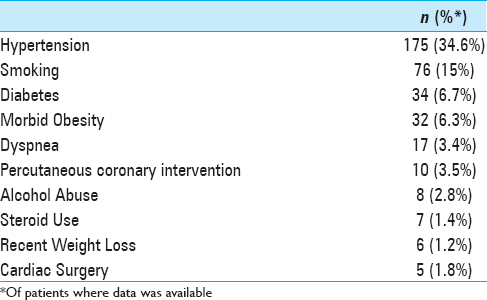
Demographics and medical comorbidities were reviewed. Body mass index (BMI) was calculated from height and weight data and morbid obesity was defined as BMI >40. The American Society of Anesthesiology (ASA) physical status classification was binned into groups 1–2 and >3 to classify patient fitness prior to surgery. Comorbidities were only analyzed if present in at least five patients, and data points must have been available in at least half of the patients to be considered for analysis. When complete data was not available, percentile values were calculated from the proportion of patients where the presence or absence of the comorbidity was recorded.
Outcome measures
Thirty-day readmission, return to the operating room, and death were the primary outcomes measured. Specific ICD-9 codes associated with each readmission as well as CPT codes for each reoperation were categorized and recorded. Medical complications and length of postoperative hospital stay were also noted.
Statistical analysis
Two-tailed Student's t-tests were performed for continuous variables, whereas Pearson's Chi-squared tests, analysis of variance, or Fisher's exact tests were used to compare proportions of categorical data or diagnoses with one another. Univariate analysis of risk factors for readmission or reoperation was performed for demographic variables, including age, sex, ASA class, and comorbidities. Statistically significant values were identified with a P value of less than 0.05, and confidence intervals were defined at 95%.
Multivariate logistic regression models were performed to evaluate predictors of readmission or reoperation. All demographic variables, indication for operation, and comorbidities with a P value less than 0.1 on univariate analysis were included in multivariate analysis. Statistics were calculated using SPSS (IBM Corporation, Armonk, NY).
RESULTS
Demographics
Five hundred and six craniotomies were reviewed. Demographic data is outlined in
Clinical outcomes
Patient outcomes are outlined in
Risk factors for readmission and reoperation
Analysis of risk factors for readmission and reoperation are illustrated in
Need for reoperation was associated with diabetes (P = 0.011) and approached significance with morbid obesity (P = 0.052). On multivariate analysis, both diabetes (P = 0.017; OR 6.32; CI 1.39–28.70) and morbid obesity (P = 0.030; OR 5.26; CI 1.17–23.59) were significant risk factors for reoperation. Data on reoperations with CPT code were available in 9/14 patients in the cohort [
DISCUSSION
TN, HFS, and GN are episodic, debilitating craniofacial syndromes. MVD is a nondestructive surgical procedure that is both common and highly effective in refractory cases, with complete pain relief rates of 76–98% reported.[
A challenge of investigating surgeries with low risk profiles is that often very large samples are necessary to capture complications. Given the relatively safe nature of MVD procedures, risk factors predisposing to complications have been poorly identified. ACS-NSQIP is a national database gaining acceptance as a tool in quality improvement and reducing complications.[
Readmissions and reoperations
The findings of the NSQIP cohort reaffirm MVD as a relatively safe procedure. With an overall readmission rate of 5.5%, reoperation rate of 2.8%, and no deaths, these findings are similar to slightly better than elsewhere reported in the literature.[
Need for repeat surgery is uncommon following MVD. A recent literature review of MVD complications by Bartek et al,[
Risk factors
In the NSQIP cohort, neither age and sex nor indication for surgery were associated with risk of readmission or reoperation. The relatively low overall complication overall and lack of significance of age support MVD as a relatively safe procedure suitable for most patients, including the elderly. These findings are reinforced by a recent comparison of elderly and nonelderly patients which identified equally effective outcomes following surgery and no significant differences in hospital stay or complications.[
Both diabetes and morbid obesity in the NSQIP cohort were found to be significantly associated with risk of readmission and reoperation, with risk of reoperation remaining significant on multivariate analysis. A higher complication rate in diabetic patients has been observed in other surgical specialties as well, including breast reconstruction and head and neck surgery.[
Avoiding readmission and reoperations
Although uncommon, readmissions and reoperations after MVD occur. How can they be prevented? Postoperatively, expedient discharge is preferable to avoid medical complications seen in any hospital admission, including urinary tract infections, pneumonia, and deep venous thrombosis (DVT). While neurologic monitoring in the immediate postoperative setting is necessary, a recent study revealed that patients without postoperative ICU stay after MVD had the same number of complications as patients sent to the ICU but had a significantly shorter length of stay and hospital cost.[
While diabetes and morbid obesity are preoperative characteristics that cannot be controlled on admission, additional care in patients with these risk factors may help avoid adverse outcomes. Meticulous dural closure and complete cranial defect reconstruction,[
Limitations
Studies of the NSQIP cohort include several limitations. The NSQIP, by design, collects data relevant to most surgical patients, including rates of readmission, reoperation, and death, but does not include several variables of interest for neurosurgery patients, including Karnofsky performance status on discharge or improvement of symptoms. The success rate for MVD in the short term, however, is very high, with reoperations for recurrent symptoms extremely uncommon, a finding observed in the NSQIP cohort. Data for the NSQIP MVD cohort is also partially incomplete, with most data on patient readmissions absent before 2011. Hospital MVD volume, which is associated with complication rate,[
Despite these limitations, however, the NSQIP avoids the inherent biases of single institution or single surgeon series. The NSQIP, by design, is a composite of data from hundreds of hospitals throughout the world, including academic and community centers, making it fairly representative of the neurosurgical community as a whole. The NSQIP further affords a sufficient sample size of patients for detailed statistical analysis in populations even with a low complication rate such as MVD. This cohort of patients collected over a relatively short time frame, therefore, provides an adequate “snapshot” of current management for MVD.
CONCLUSIONS
MVD is an effective and commonly performed procedure in neurosurgery and forms an essential part of treatment for refractory TN, HFS, and GN. In this NSQIP cohort, a 30-day readmission rate of 5.5% and reoperation rate of 2.8% were identified, reaffirming the relative safety of contemporary surgery for TN, HFS, and GN.
Although safe, risk of complications after MVD persist despite optimal surgical management. Diabetes and morbid obesity were significantly associated with risk of reoperation, and approached significance for readmission. Age, sex, ASA class, and indication for surgery were not associated with poor outcomes. While further research is needed to identify the optimal strategy to reduce readmissions and repeat surgery, additional care in patients with these risk factors may help avoid adverse outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bartek J, Gulati S, Unsgard G, Weber C, Forander P, Solheim O. Standardized reporting of adverse events after microvascular decompression of cranial nerves; a population-based single-institution consecutive series. Acta Neurochir. 2016. 158: 1775-81
2. Berger I, Nayak N, Schuster J, Lee J, Stein S, Malhotra NR. Microvascular Decompression Versus Stereotactic Radiosurgery for Trigeminal Neuralgia: A Decision Analysis. Cureus. 2017. 9: e1000-
3. Bick SKB, Eskandar EN. Surgical Treatment of Trigeminal Neuralgia. Neurosurg Clin N Am. 2017. 28: 429-38
4. Brisman R. Microvascular decompression vs. gamma knife radiosurgery for typical trigeminal neuralgia: Preliminary findings. Stereotact Funct Neurosurg. 2007. 85: 94-8
5. Burchiel KJ, Clarke H, Haglund M, Loeser JD. Long-term efficacy of microvascular decompression in trigeminal neuralgia. J Neurosurg. 1988. 69: 35-8
6. Chaudhry N, Srivastava A, Joshi L. Hemifacial spasm: The past, present and future. J Neurol Sci. 2015. 356: 27-31
7. Chen J, Sindou M. Vago-glossopharyngeal neuralgia: A literature review of neurosurgical experience. Acta Neurochir. 2015. 157: 311-21
8. da Silva OT, de Almeida CC, Iglesio RF, de Navarro JM, Teixeira MJ, Duarte KP. Surgical variation of microvascular decompression for trigeminal neuralgia: A technical note and anatomical study. Surg Neurol Int. 2016. 7: S571-6
9. Dai ZF, Huang QL, Liu HP, Zhang W. Efficacy of stereotactic gamma knife surgery and microvascular decompression in the treatment of primary trigeminal neuralgia: A retrospective study of 220 cases from a single center. J Pain Res. 2016. 9: 535-42
10. Dasenbrock HH, Yan SC, Smith TR, Valdes PA, Gormley WB, Claus EB. Readmission After Craniotomy for Tumor: A National Surgical Quality Improvement Program Analysis. Neurosurgery. 2017. 80: 551-62
11. Haines SJ, Jannetta PJ, Zorub DS. Microvascular relations of the trigeminal nerve. An anatomical study with clinical correlation. J Neurosurg. 1980. 52: 381-6
12. Hart A, Funderburk CD, Chu CK, Pinell-White X, Halgopian T, Manning-Geist B. The Impact of Diabetes Mellitus on Wound Healing in Breast Reconstruction. Ann Plast Surg. 2017. 78: 260-3
13. Higgins DM, Mallory GW, Planchard RF, Puffer RC, Ali M, Gates MJ. Understanding the Impact of Obesity on Short-term Outcomes and In-hospital Costs After Instrumented Spinal Fusion. Neurosurgery. 2016. 78: 127-32
14. Huh R, Han IB, Moon JY, Chang JW, Chung SS. Microvascular decompression for hemifacial spasm: Analyses of operative complications in 1582 consecutive patients. Surg Neurol. 2008. 69: 153-7
15. Jannetta PJ. Cranial nerve vascular compression syndromes (other than tic douloureux and hemifacial spasm). Clin Neurosurg. 1981. 28: 445-56
16. Jones RE, Russell RD, Huo MH. Wound healing in total joint replacement. Bone Joint J. 2013. 95-b: 144-7
17. Kalkanis SN, Eskandar EN, Carter BS, Barker FG. Microvascular decompression surgery in the United States, 1996 to 2000: Mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery. 2003. 52: 1251-
18. Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990. 27: 89-95
19. Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell DA. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: The Patient Safety in Surgery study. Ann Surg. 2008. 248: 329-36
20. Lawrence JD, Tuchek C, Cohen-Gadol AA, Sekula RF. Utility of the intensive care unit in patients undergoing microvascular decompression: A multiinstitution comparative analysis. J Neurosurg. 2016. p.
21. Linskey ME, Ratanatharathorn V, Penagaricano J. A prospective cohort study of microvascular decompression and Gamma Knife surgery in patients with trigeminal neuralgia. J Neurosurg. 2008. 109: 160-72
22. Montava M, Rossi V, CurtoFais CL, Mancini J, Lavieille JP. Long-term surgical results in microvascular decompression for hemifacial spasm: Efficacy, morbidity and quality of life. Acta Otorhinolaryngol Ital. 2016. 36: 220-7
23. Montroy J, Breau RH, Cnossen S, Witiuk K, Binette A, Ferrier T. Change in Adverse Events After Enrollment in the National Surgical Quality Improvement Program: A Systematic Review and Meta-Analysis. PloS One. 2016. 11: e0146254-
24. Nanda A, Javalkar V, Zhang S, Ahmed O. Long term efficacy and patient satisfaction of microvascular decompression and gamma knife radiosurgery for trigeminal neuralgia. J Clin Neurosci. 2015. 22: 818-22
25. O’Connor JK, Bidiwala S. Effectiveness and safety of Gamma Knife radiosurgery for glossopharyngeal neuralgia. Proceedings (Baylor University Medical Center). 2013. 26: 262-4
26. Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: Analysis of 217 cases. Neurosurgery. 2002. 50: 705-
27. Raikundalia MD, Fang CH, Spinazzi EF, Vazquez A, Park RC, Baredes S. Impact of Diabetes Mellitus on Head and Neck Cancer Patients Undergoing Surgery. Otolaryngol Head Neck Surg. 2016. 154: 294-9
28. Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995. 36: 64-
29. Sampson JH, Grossi PM, Asaoka K, Fukushima T. Microvascular decompression for glossopharyngeal neuralgia: Long-term effectiveness and complication avoidance. Neurosurgery. 2004. 54: 884-
30. Sandel T, Eide PK. Long-term results of microvascular decompression for trigeminal neuralgia and hemifacial spasms according to preoperative symptomatology. Acta Neurochir. 2013. 155: 1681-92
31. Sekula RF, Frederickson AM, Jannetta PJ, Quigley MR, Aziz KM, Arnone GD.. Microvascular decompression for elderly patients with trigeminal neuralgia: A prospective study and systematic review with meta-analysis. J Neurosurg. 2011. 114: 172-9
32. Stanic S, Franklin SD, Pappas CT, Stern RL. Gamma knife radiosurgery for recurrent glossopharyngeal neuralgia after microvascular decompression. Stereotact Funct Neurosurg. 2012. 90: 188-91
33. Stoker MA, Forbes JA, Hanif R, Cooper C, Nian H, Konrad PE. Decreased Rate of CSF Leakage Associated with Complete Reconstruction of Suboccipital Cranial Defects. J Neurol Surg Part B Skull Base. 2012. 73: 281-6
34. Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: Literature study of respective long-term outcomes. Acta Neurochir. 2008. 150: 243-55
35. Wang DD, Ouyang D, Englot DJ, Rolston JD, Molinaro AM, Ward M. Trends in surgical treatment for trigeminal neuralgia in the United States of America from 1988 to 2008. J Clin Neurosci. 2013. 20: 1538-45
36. Xia L, Zhong J, Zhu J, Wang YN, Dou NN, Liu MX. Effectiveness and safety of microvascular decompression surgery for treatment of trigeminal neuralgia: A systematic review. J Craniofac Surg. 2014. 25: 1413-7