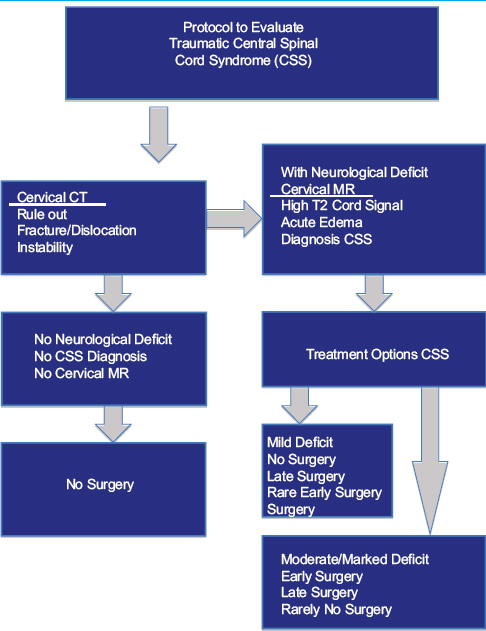
- Department of NeuroScience, Winthrop University Hospital, Mineola, NY 11501, USA
Correspondence Address:
Nancy E. Epstein
Department of NeuroScience, Winthrop University Hospital, Mineola, NY 11501, USA
DOI:10.4103/2152-7806.156552
Copyright: © 2015 Epstein NE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Epstein NE, Hollingsworth R. Diagnosis and management of traumatic cervical central spinal cord injury: A review. Surg Neurol Int 07-May-2015;6:
How to cite this URL: Epstein NE, Hollingsworth R. Diagnosis and management of traumatic cervical central spinal cord injury: A review. Surg Neurol Int 07-May-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/diagnosis-management-traumatic-cervical-central-spinal/
Abstract
Background:The classical clinical presentation, neuroradiographic features, and conservative vs. surgical management of traumatic cervical central spinal cord (CSS) injury remain controversial.
Methods:CSS injuries, occurring in approximately 9.2% of all cord injuries, are usually attributed to significant hyperextension trauma combined with congenital/acquired cervical stenosis/spondylosis. Patients typically present with greater motor deficits in the upper vs. lower extremities accompanied by patchy sensory loss. T2-weighted magnetic resonance (MR) scans usually show hyperintense T2 intramedullary signals reflecting acute edema along with ligamentous injury, while noncontrast computed tomography (CT) studies typically show no attendant bony pathology (e.g. no fracture, dislocation).
Results:CSS constitute only a small percentage of all traumatic spinal cord injuries. Aarabi et al. found CSS patients averaged 58.3 years of age, 83% were male and 52.4% involved accidents/falls in patients with narrowed spinal canals (average 5.6 mm); their average American Spinal Injury Association (ASIA) motor score was 63.8, and most pathology was at the C3-C4 and C4-C5 levels (71%). Surgery was performed within 24 h (9 patients), 24–48 h (10 patients), or after 48 h (23 patients). In the Brodell et al. study of 16,134 patients with CSS, 39.7% had surgery. In the Gu et al. series, those with CSS and stenosis/ossification of the posterior longitudinal ligament (OPLL) exhibited better outcomes following laminoplasty.
Conclusions:Recognizing the unique features of CSS is critical, as the clinical, neuroradiological, and management strategies (e.g. conservative vs. surgical management: early vs. late) differ from those utilized for other spinal cord trauma. Increased T2-weighted MR images best document CSS, while CT studies confirm the absence of fracture/dislocation.
Keywords: Cervical, central cord injury, conservative therapy, spinal trauma, surgery
INTRODUCTION
Traumatic cervical central cord spinal injuries (CSS) are now more readily recognized both clinically and on magnetic resonance (MR) scans. McKinley found that 9.2% of all spinal cord injuries were attributed to central spinal cord syndromes (CSSs; 77 of 839).[
DEFINITION OF CENTRAL CORD SYNDROME
Central cord syndrome
Nowak et al. considered CSS to be the most common type of incomplete spinal cord injury that typically occurs following traumatic hyperextension events in older patients with underlying cervical spondylosis/stenosis [
Epidemiology of severe cervical spinal trauma in the north area of são paulo city
Santos et al. evaluated cervical spinal trauma (CST) data over a 10-year period, 1997–2006 [
INJURY MECHANISMS AND DAMAGE AFTER ACUTE CENTRAL CORD SYNDROMES: ANIMAL MODEL
Acute central cord syndrome: Injury mechanisms
Li and Dai assessed the stress distribution to the cervical cord under different injury conditions to better understand the etiology of acute CCSs [
ETIOLOGY OF CENTRAL CORD INJURIES
CSS: Traumatic myelopathy in patients with stenosis without fracture/dislocation
Epstein et al., in 1980, published one of the first articles observing the incidence of traumatic myelopathy occurring in patients with cervical spinal stenosis without fracture or dislocation [
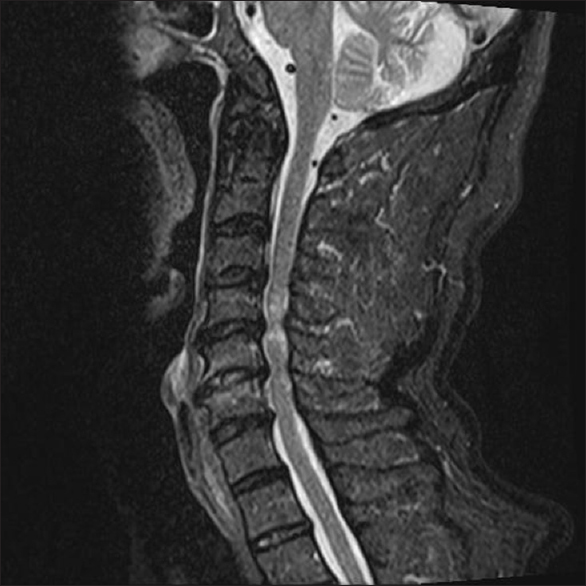
Figure 1
Midline sagittal stir T2-weighted MR image following CSS. following a hyperextension injury in a 75-year-old male, this midline sagittal stir T2-weighted MR image readily demonstrated marked intrinsic signal changes within the spinal cord opposite the C4 and C5 vertebral bodies. Note the maximal cord compression opposite the disc space of C4-C5, followed by C5-C6. At the C6-C7 level, there is thecal sac intrusion without significant cord compression
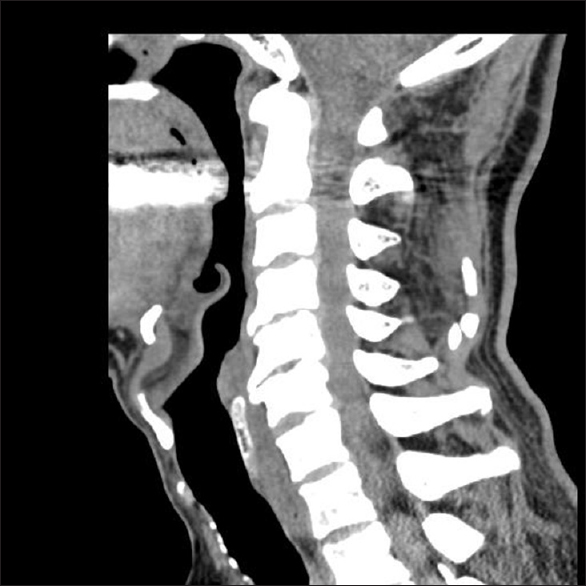
Figure 2
Midline sagittal soft-tissue window CT study following CSS. In the same 75-year-old male, following a hyperextension injury, the midline sagittal soft-tissue window CT study confirmed the absence of fracture or dislocation following the hyperextension traumatic event that led to the CSS. Note the presence of spinal stenosis/spondyosis documented by ventral osteophytes at multiple levels (e.g., most marked at C4-C5- and C5-C6, followed by C6-C7 and C3-C4) and the accompanying dorsolateral shingling of the C4, C5, C6 laminae resulting in the greatest canal compromise at the C4-C5 and C5-C6 levels
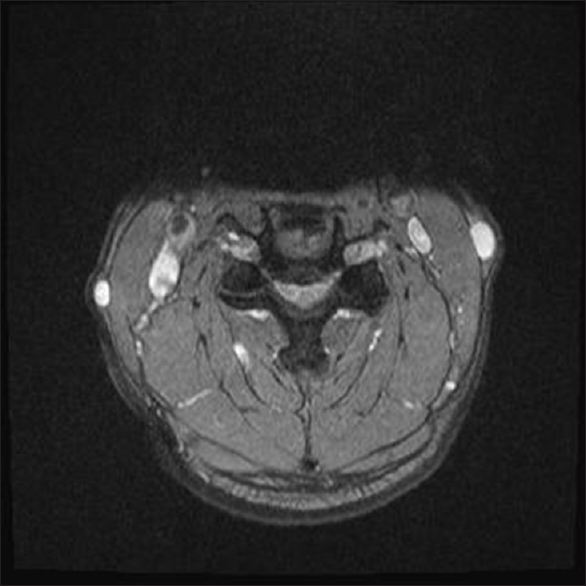
Figure 3
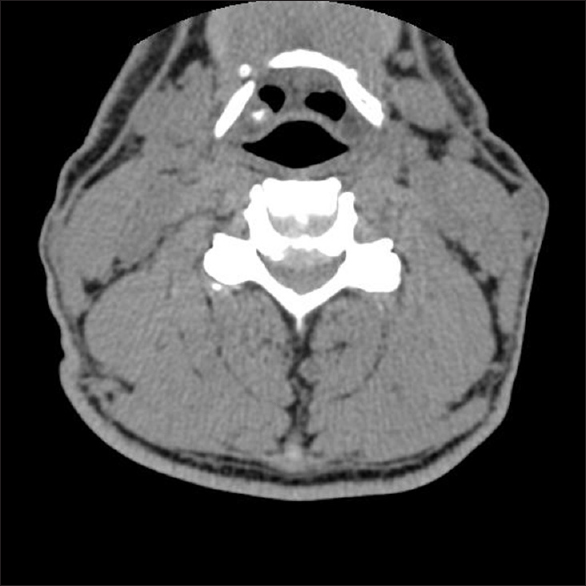
Axial T2-weighted STIR MR image at the C4-C5 disc space following the CSS. On the axial T2 weighted STIR MR image at the C4-C5 disc space level following the CSS one can see the marked reduction in the anterior-posterior diameter of the spinal canal to 5–6 mm secondary to ventral osteophyte formation vs. disc, and dorsal laminar shingling. On this STIR study, there also appears to be an increased signal in the cord at this level
Figure 4
Axial CT image at the C4-C5 disc space following the CSS. This axial noncontrast CT study demonstrated marked stenosis/ spondylosis at the C4-C5 level narrowing down the spinal canal to 5–6 mm. Ventrally the hyperdense soft-tissue appears more spondylotic vs. soft disc, while posteriorly the compression was attributed to inward shingling of the C5 lamina. Certainly, both the MR and CT studies help document why this patient, with such severe stenosis at the C4-C5 level, was so susceptible to the CSS cord injury
Figure 5
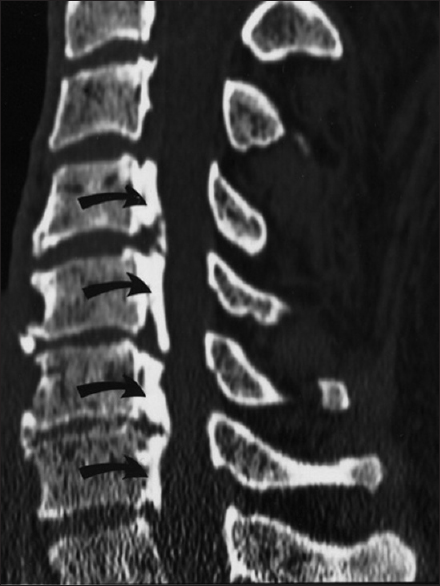
Ossification of the posterior longitudinal ligament (OPLL) contributing to cervical Stenosis. Patient with severe OPLL and markedly narrowed cervical spinal canals are very susceptible to CSS injury following even minor hyperextension traumatic events. Note the segmental/continuous forms of OPLL extending from the C4-C7 vertebral levels that lead to a reduced AP diameter of the canal to less than 5 mm at multiple foci
Figure 6
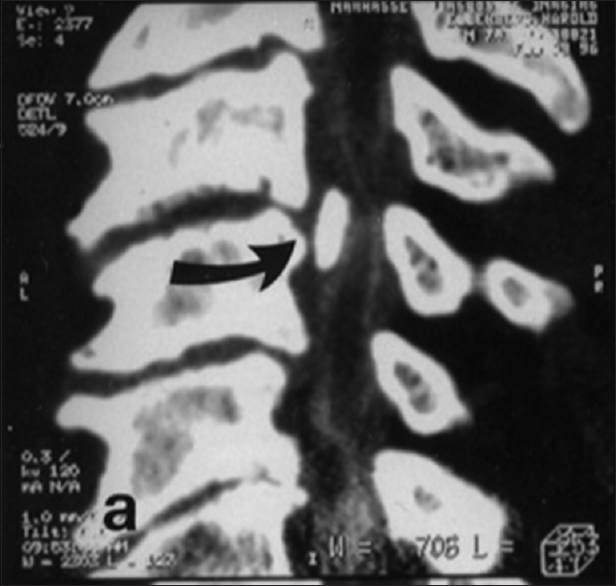
Midline sagittal Myelo-CT documenting early OPLL compressing the cord at the C3-C4 and C4-C5 Levels. Patient with marked cord compression due to early OPLL are also extremely susceptible to CSS injury following even minor hyperextension trauma. Early OPLL is defined by hypertrophied posterior longitudinal ligament (HPLL) combined with progressive punctate ossification centers. In this midline sagittal Myelo-CT study, marked ventral compression at the C3-C4 level is attribute to a coalescence of punctate ossification centers within HPLL (e.g., pearl of ossification). At the C4-C5 level below, HPLL itself without such ossification contributes to marked cord compromise
Central cord injury: Pathophysiology, management, and outcomes
Harrop et al. utilized Medline to evaluate the pathophysiology, management, and outcomes of acute traumatic central cord syndromes (ATCCSs)/injuries (CSS) [
Pathoanatomic etiologies of ATCCS injuries were variously attributed to: Hematomyelia (worse injury/poor prognostic finding), vascular insult/ischemia (vertebral compression), or mechanical injury. T2-weighted MR studies typically demonstrate hyperintense signals in the cord tissues involving the lateral white matter tracts (corticospinal) with accompanying axonal and myelin loss (the latter confirmed on autopsy).
Acute traumatic CSS; clinical and radiological correlations
Miranda et al. correlated neurological impairment and radiological findings for 15 patients who sustained acute traumatic CSS [
Hyperextension trauma leads to central cord syndrome
Aarabi et al. evaluated traumatic central cord syndromes (TCCSs) characterized by incomplete spinal cord injuries [
Hyperextension CSS injury of the cervical spine and outcomes
Thompson et al. noted that TCCS is the most common form of an incomplete SCI [
Cervical spine injuries in ocean bathers: Wave-related accidents
Robles evaluated spine injuries resulting from severe aquatic recreational activities; for example, hyperextension injuries resulting in CCSs [
Pediatric central cord injuries (child abuse)
Feldman et al. studied the mechanism of injury in five infants/toddlers who sustained cervical spinal cord injuries (CSSs) associated with brain injuries resulting in paralysis [
CSS after adequate decompression for cervical spondylosis
Dickerman et al. presented a case in which a patient developed a CSS following a decompressive C3-C7 cervical laminectomy for cervical stenosis [
Central cord injury with Klippel–Feil syndrome
Epstein et al. discussed the onset of traumatic myelopathy attributed to a hyperextension injury incurred when a 17-year-old male with a C2-C3 Klippel–Feil (KL) congenital fusion/stenosis was body surfing [
OTHER ETIOLOGIES OF CENTRAL CORD INJURIES
CSS-related status epilepticus: A case report
In this case report, Lee et al. noted that a 25-year-old male sustained a CCS after status epilepticus [
CSS after total hip replacement
Buchowski et al. presented a case in which a patient sustained a CSS following a total hip replacement performed under general endotracheal anesthesia [
Acute/subacute spinal epidural hematoma resulting in CSS
Yu et al. looked at the diagnosis/treatment of spinal epidural hematomas in 11 patients; 2 had clots in the cervical spine, and 2 at the cervico-thoracic junction [
DIFFERENTIAL DIAGNOSES OF CENTRAL CORD SYNDROMES
Devic's syndrome: Initial presentation of systemic lupus erythematosus
Karim and Majithia defined Devic's syndrome/neuromyelitis optica (NMO) as an “inflammatory demyelinating disease of the central nervous system (CNS) associated with optic neuritis.”[
Primary sjogren's syndrome involving the central nervous system
Alhomoud et al. described the clinical, laboratory, and radiological features of primary Sjogren's syndrome (PSS) presenting within the CNS [
Multiple sclerosis: Mri-defined spinal cord involvement/disability in ms
For multiple sclerosis (MS) patients, Cohen et al. correlated MRI-defined lesions, atrophy, and other pathology reflecting brain/spinal cord involvement with disability [
RADIOGRAPHIC STUDIES CORRELATION WITH CSS
Hyperintense T2 intramedullary MR signal correlates with CSS
Song et al. assessed the clinical/prognostic value of dynamic X-rays and MR studies in 23 patients with CCS without fracture/dislocation [
Cervical spine trauma clearance: Are multidetector CT scans sufficient?
Chew et al. observed that clearing the cervical spine following trauma is critical.[
Acute CSS: Rare anterior spinal artery syndromes on CT angiography
Zhang et al. observed that with acute traumatic SCI significant vascular damage may be associated with spinal contusions, fractures, and dislocations [
SCORING SYSTEM FOR SPINAL CORD INJURY
Cervical injury scoring using a subaxial injury classification system
Joaquim et al. noted that the subaxial injury classification (SLIC) system and severity score facilitates the management of subaxial cervical spine injuries [
MEDICAL COMPLICATIONS OF CENTRAL CORD SYNDROME
Bilateral upper-extremity deep vein thrombosis after CSS
Onmez et al. evaluated the incidence of deep vein thrombosis (DVT) involving the upper extremities in patients who sustained SCI characterized by CCS [
CONSERVATIVE VS. SURGICAL MANAGEMENT OF CSS
Conservative treatment vs. Surgery for traumatic central cord syndrome
Stevens et al. reviewed the recommendations for conservative treatment (59 patients) vs. surgery (67 patients), timing of surgery (<24 h, >24 h, or delayed second admission), outcomes (Frankel Grade), and length of stay (LOS; intensive care unit) for 126 patients who sustained TCSSs between 1985 and 2006 [Tables
Trends in management of central cord syndrome in 16,134 patients
Brodell et al. evaluated the treatment (surgical vs. nonsurgical) for 16,134 patients following traumatic cervical CCSs [
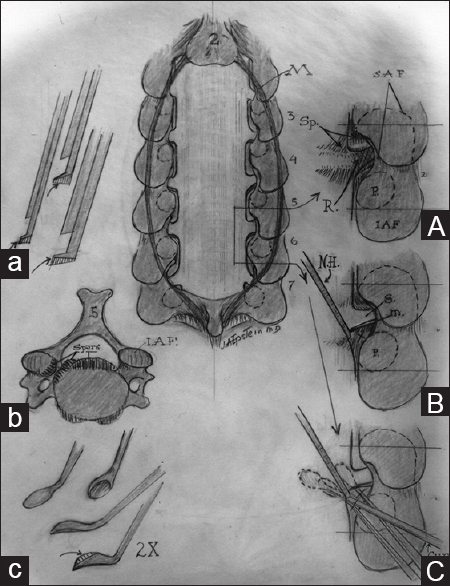
Figure 7
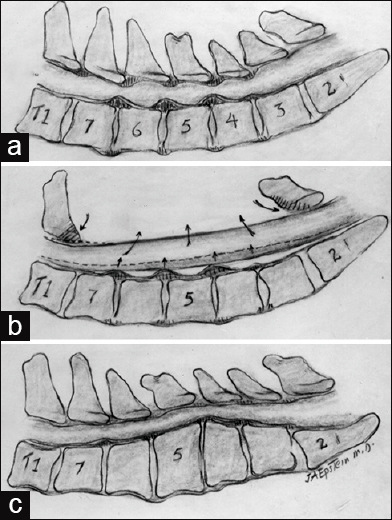
Patients with cervical spinal stenosis are susceptible to CSS and may require posterior or anterior decompressive surgery (a) This midline lateral illustration of cervical stenosis demonstrates diffuse ventral spondylotic intrusions at the C4-C5, C5-C6, and C6- C7 levels and dorsolateral compression attributed to multilevel inward buckling of hypertrophied/ossified yellow ligament (OYL) and inward shingling of the lamina. (b) When the cervical lordotic curvature is adequately preserved, a dorsal decompression with/ without fusion may sufficiently decompress the spinal cord. Illustrated here is a laminectomy involving resection of the C3-C7 laminae. (c) In this instance, there reversal of the lordosis with substantial kyphosis warranting a multilevel anterior decompressive procedure (e.g., corpectomies)
Figure 8
Multilevel laminectomy with medial facetectomy/ foraminotomy. CENTRAL IMAGE: This illustrates the posterior view of a C3-C7 laminectomy accompanied by medial facetectomy/ foraminotomy at the C23, C34, C45, C56, C67 C7T1 levels. (a) Illustration of filed-down Kerrison Rongeur utilized to perform medial facetectomy/foraminotomy and resect hypertrophied/ ossified yellow ligament to decompress the nerve roots in the foramina. (b) Axial illustration of the ventral/foraminal osteophytes, which often require resection with a down-biting curette.(c) Illustration of the down-biting curette employed to remove foraminal disc/spur/osteophytes. (A) Illustration of nerve root exiting into the foramen following performance of a medial facetectomy/foraminotomy. (B) In order to resect foraminal disc or spur, utilizing a micro nerve hook allows for mobilization of the foraminally exiting nerve root. (C)The down-biting curette is then introduced ventral to the root and lateral aspect of the thecal sac to remove disc/spur
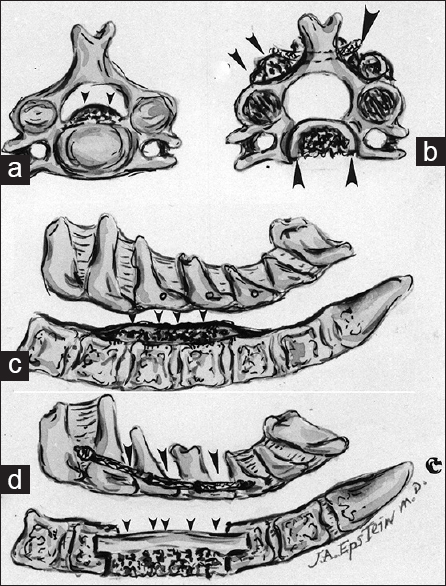
Figure 9
Illustrations of multilevel OPLL before after multilevel anterior cervical corpectomy/fusion.(a) Axial image of ventral OPLL filling the midline spinal canal (small arrows).(b) Axial image following anterior corpectomy and fusion (ventral graft in place) with posterior wires/spinous process fusion (single arrow) and accompanying bone graft (double arrows).(c) Midline sagittal illustration of multilevel continuous OPLL starting behind the inferior C4 and extending to the superior aspect of the C7 vertebral body.(d) Illustration of an anterior fibular strut graft extending from C4-C7 with posterior wiring and fusion involving the same levels
Laminoplasty vs. Conservative treatment of CSS due to OPLL
Gu et al. evaluated how patients who sustained acute spinal cord injuries following minor trauma (falls, motor vehicle accidents, sports) with underlying ossification of the posterior longitudinal ligament (OPLL: Mostly with mixed and segmental variants) were managed conservatively (C group; 29 patients) or surgically (L group – lamionplasty; 31 patients) [
Acute traumatic CSS treated with open-door expansile cervical laminoplasty
Uribe et al. evaluated the efficacy of open-door expansile cervical laminoplasty (ODECL) in the management of CSS attributed to multilevel cervical spondylotic myelopathy [
Surgery for acute subaxial traumatic CSS without fracture or dislocation
Song et al. evaluated 22 patients with subaxial acute traumatic CCS without fracture or dislocation who required spinal surgery [
TIMING OF SURGERY AND OUTCOMES FOR CSS
Outcomes for acute traumatic central cord syndrome due to spinal stenosis
Aarabi et al. evaluated multiple variables impacting the 1-year postoperative outcomes for patients sustaining ATCCSs attributed to spinal stenosis [
Urgency of early surgical decompression in acute CSS with stenosis
Lenehan et al. evaluated whether urgent surgical decompression facilitates neurologic recovery following acute central cord injuries without attendant fracture/dislocation [Tables
Early surgery for spinal cord injury, especially for incomplete CSS
Fehlings et al. evaluated the optimal timing of surgery (early vs. later decompression) for acute SCI [
Incidence and outcomes of acute CSS and other clinical syndromes
McKinley et al. examined the factors correlating with outcomes for patients sustaining SCI associated with CSS, BSS, anterior cord syndrome (ACS), posterior cord syndrome (PCS), cauda equina syndrome (CES), and conus medullaris syndrome (CMS) [
Factors predicting motor recovery and outcome after traumatic CSS
For patients who sustained traumatic CSS, Dvorak et al. prospectively assessed improvement in AMS (at 72 h and follow-up), generic health-related quality of life (HRQoL), and functional status [
SUMMARY
Although the treatment strategies for CSS remain controversial, establishing the diagnosis of CSS has been greatly facilitated with the ready availability of CT and MR studies [Tables
References
1. Aarabi B, Koltz M, Ibrahimi D. Hyperextension cervical spine injuries and traumatic central cord syndrome. Neurosurg Focus. 2008. 25: E9-
2. Aarabi B, Alexander M, Mirvis SE, Shanmuganathan K, Chesler D, Maulucci C. Predictors of outcome in acute traumatic central cord syndrome due to spinal stenosis. J Neurosurg Spine. 2011. 14: 122-30
3. Alhomoud IA, Bohlega SA, Alkawi MZ, Alsemari AM, Omer SM, Alsenani FM. Primary Sjogren's syndrome with central nervous system involvement. Saudi Med J. 2009. 30: 1067-72
4. Brodell DW, Jain A, Elfar JC, Mesfin A. National Trends in the Management of Central Cord Syndrome: An Analysis of 16,134 patients. Spine J. 2015. 15: 435-42
5. Buchowski JM, Kebaish KM, Suk KS, Kostuik JP. Central cord syndrome after total hip arthroplasty: A patient report. Spine (Phila Pa 1976). 2005. 30: E103-5
6. Chew BG, Swartz C, Quigley MR, Altman DT, Daffner RH, Wilberger JE. Cervical spine clearance in the traumatically injured patient: Is multidetector CT scanning sufficient alone? Clinical article. J Neurosurg Spine. 2013. 19: 576-81
7. Cohen AB, Neema M, Arora A, Dell’oglio E, Benedict RH, Tauhid S. The relationships among MRI-defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. J Neuroimaging. 2012. 22: 122-8
8. Dickerman RD, Lefkowitz M, Epstein JA. A traumatic central cord syndrome occurring after adequate decompression for cervical spondylosis: Biomechanics of injury: Case report. Spine (Phila Pa 1976). 2005. 30: E611-3
9. Dvorak MF, Fisher CG, Hoekema J, Boyd M, Noonan V, Wing PC. Factors predicting motor recovery and functional outcome after traumatic central cord syndrome: A long-term follow-up. Spine (Phila Pa 1976). 2005. 30: 2303-11
10. Epstein NE, Epstein JA, Benjamin V, Ransohoff J. Traumatic myelopathy in patients with cervical spinal stenosis without fracture or dislocation: Methods of diagnosis, management, and prognosis. Spine. 1980. 6: 489-96
11. Epstein NE, Epstein JA, Zilkha A. Traumatic myelopathy in a seventeen-year-old child with cervical spinal stenosis (without fracture or dislocation) and a C2-C3 Klippel-Feil fusion. A case report. Spine (Phila Pa 1976). 1984. 9: 344-7
12. Fehlings MG, Rabin D, Sears W, Cadotte DW, Aarabi B. Current practice in the timing of surgical intervention in spinal cord injury. Spine (Phila Pa 1976). 2010. 35: S166-73
13. Feldman KW, Avellino AM, Sugar NF, Ellenbogen RG. Cervical spinal cord injury in abused children. Pediatr Emerg Care. 2008. 24: 222-7
14. Gu Y, Chen L, Dong RB, Feng Y, Yang HL, Tang TS. Laminoplasty versus conservative treatment for acute cervical spinal cord injury caused by ossification of the posterior longitudinal ligament after minor trauma. Spine J. 2014. 14: 344-52
15. Harrop JS, Sharan A, Ratliff J. Central cord injury: Pathophysiology, management, and outcomes. Spine J. 2006. 6: 198-206S
16. Joaquim AF, Patel AA, Vaccaro AR, Joaquim AF, Patel AA, Vaccaro AR. Cervical injuries scored according to the Subaxial Injury Classification system: An analysis of the literature. J Craniovertebr Junction Spine. 2014. 5: 65-70
17. Karim S, Majithia V. Devic's syndrome as initial presentation of systemic lupus erythematosus. Am J Med Sci. 2009. 338: 245-7
18. Lee S, Lee JE, Yang S, Chang H. A case of central cord syndrome related status epilepticus-a case report. Ann Rehabil Med. 2011. 35: 574-8
19. Lenehan B, Fisher CG, Vaccaro A, Fehlings M, Aarabi B, Dvorak MF. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976). 2010. 35: S180-6
20. Li XF, Dai LY. Acute central cord syndrome: Injury mechanisms and stress features. Spine (Phila Pa 1976). 2010. 35: E955-64
21. McKinley W, Santos K, Meade M, Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007. 30: 215-24
22. Miranda P, Gomez P, Alday R. Acute traumatic central cord syndrome: Analysis of clinical and radiological correlations. J Neurosurg Sci. 2008. 52: 107-12
23. Nowak DD, Lee JK, Gelb DE, Poelstra KA, Ludwig SC. Central cord syndrome. J Am Acad Orthop Surg. 2009. 17: 756-65
24. Onmez H, Cingoz HT, Kucuksen S, Anliacık E, Yaşar O, Yilmaz H. Bilateral upper-extremity deep vein thrombosis following central cord syndrome. J Spinal Cord Med. 2013. 36: 243-6
25. Robles LA. Cervical spine injuries in ocean bathers: Wave-related accidents. Neurosurgery. 2006. 58: 920-3
26. Santos EA, Filho WJ, Possatti LL, Bittencourt LR, Fontoura EA, Botelho RV. Epidemiology of severe cervical spinal trauma in the north area of São Paulo City: A 10-year prospective study. Clinical article. J Neurosurg Spine. 2009. 11: 34-41
27. Song J, Mizuno J, Nakagawa H, Inoue T. Surgery for acute subaxial traumatic central cord syndrome without fracture or dislocation. J Clin Neurosci. 2005. 12: 438-43
28. Song J, Mizuno J, Inoue T, Nakagawa H. Clinical evaluation of traumatic central cord syndrome: Emphasis on clinical significance of prevertebral hyperintensity, cord compression, and intramedullary high-signal intensity on magnetic resonance imaging. Surg Neurol. 2006. 65: 117-23
29. Stevens EA, Marsh R, Wilson JA, Sweasey TA, Branch CL, Powers AK. A review of surgical intervention in the setting of traumatic central cord syndrome. Spine J. 2010. 10: 874-80
30. Thompson C, Gonsalves JF, Welsh D. Hyperextension injury of the cervical spine with central cord syndrome. Eur Spine J. 2015. 24: 195-202
31. Uribe J, Green BA, Vanni S, Moza K, Guest JD, Levi AD. Acute traumatic central cord syndrome-experience using surgical decompression with open-door expansile cervical laminoplasty. Surg Neurol. 2005. 63: 505-10
32. Yu HP, Fan SW, Yang HL, Tang TS, Zhou F, Zhao X. Early diagnosis and treatment of acute or subacute spinal epidural hematoma. Chin Med J (Engl). 2007. 120: 1303-8
33. Zhang Z, Wang H, Zhou Y, Wang J. Computed tomographic angiography of anterior spinal artery in acute cervical spinal cord injury. Spinal Cord. 2013. 51: 442-7