- Neuroradiological Clinic, Neurocenter, Klinikum Stuttgart, Germany
- Neurosurgical Clinic, Neurocenter, Klinikum Stuttgart, Germany
- Neurological Clinic, Neurocenter, Klinikum Stuttgart, Germany
- Medical Faculty, University Duisburg-Essen, Germany
Correspondence Address:
P. Bhogal
Neuroradiological Clinic, Neurocenter, Klinikum Stuttgart, Germany
DOI:10.4103/sni.sni_339_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: P. Bhogal, M. AlMatter, V. Hellstern, O. Ganslandt, H. Bäzner, H. Henkes, M. Aguilar Pérez. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. 10-Jan-2018;9:1
How to cite this URL: P. Bhogal, M. AlMatter, V. Hellstern, O. Ganslandt, H. Bäzner, H. Henkes, M. Aguilar Pérez. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. 10-Jan-2018;9:1. Available from: http://surgicalneurologyint.com/surgicalint-articles/difference-in-aneurysm-characteristics-between-ruptured-and-unruptured-aneurysms-in-patients-with-multiple-intracranial-aneurysms/
Abstract
Background:The risk of aneurysmal rupture is dependent upon numerous factors, however, there are inconsistencies in the results between studies, which may be due to confounding factors. This can be avoided by comparing the characteristics of ruptured and unruptured aneurysms within the same patient. We sought to analyze the aneurysm characteristics of patients with acute aneurysmal subarachnoid hemorrhage (SAH) and multiple intracranial aneurysms.
Methods:We reviewed our prospectively maintained institutional database, between 01/10/2007 and 01/01/2017, for all patients with confirmed SAH and >1 aneurysm. We recorded the size, location, and morphology and calculated secondary geometric indices such as bottleneck factor and aspect ratio.
Results:During the study period, a total of 694 patients with aneurysmal SAH were admitted to our institution. We identified 113 patients (74.3% female, average age 51.7 ± 12.3). The majority of patients had only one associate unruptured aneurysm (79.6%). The average unruptured aneurysm was 3.1 ± 1.5 mm and the average ruptured aneurysm was 5.7 ± 2.7 mm (P P 7 mm (OR, 17.74; 95% CI 4.07–77.35; P
Conclusion:Size plays an important part in determining rupture risk, however, other factors such as location and in particular morphology must also be considered. We believe that the introduction of vessel wall imaging will help to risk stratify aneurysms.
Keywords: Aneurysm morphology, aneurysm, aspect ratio, bottleneck factor, lobulation, subarachnoid hemorrhage
INTRODUCTION
Approximately 2–3% of the population harbours an unruptured intracranial aneurysm, and approximately 30% of the patients have multiple aneurysms.[
Identifying which aneurysms, regardless of size, are prone to rupture is the key to optimizing management. A variety of different factors such as aneurysm shape, size ratio, and flow angles[
An inability to control for confounding factors may cause much of the difficulty when using a case-control design when analyzing patients with ruptured aneurysms to those with unruptured aneurysms. This can be avoided by comparing the characteristics of ruptured and unruptured aneurysms within the same patient.
We sought to analyze the morphological characteristics of ruptured and unruptured aneurysms within the same patient from a single institution.
MATERIALS AND METHODS
Study population
We reviewed our prospectively maintained institutional database, which includes consecutive patients with confirmed SAH. We extracted all patients who presented with acute aneurysmal SAH confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). We included all patients between 01/10/2007 and 01/01/2017 with confirmed SAH and more than one intracranial aneurysm confirmed on diagnostic subtraction cerebral angiography (DSA). We excluded patients without confirmed aneurysmal SAH (missing CT/MRI or DSA), patients presenting with fusiform, dissecting, mycotic, and partially thrombosed aneurysms, as well as likely pseudoaneurysms secondary to trauma.
Imaging
All patients underwent either CT or MRI to confirm the presence of SAH and CT or MR angiography to confirm the presence of an aneurysm. All patients presenting with SAH underwent DSA with complete 6-vessel angiography. In addition to the standard Towne's and lateral projections, dedicated projections of all aneurysms were performed as per our standard practice.
Aneurysmal location, measurements, and morphological characteristics
The location of the ruptured and unruptured aneurysms was categorized into internal carotid artery (ICA), anterior cerebral artery territory (ACA), anterior communicating artery (AcomA), posterior communicating artery (PcomA), middle cerebral artery (MCA), vertebral artery (VA), posterior inferior cerebellar artery (PICA), basilar artery (BA), and the posterior cerebral artery (PCA).
The maximum neck width, dome width, and dome height were recorded from the DSA using standard techniques. We calculated secondary geometric indices including the aspect ratio (AR) – the ratio of the dome height to the neck width, the height to width ratio, and the bottleneck factor – the ratio of dome width to neck width.[
Aneurysm shape was categorized as spherical (if the width was at ≥80% of the dome height), lobulated (where the lobules were smooth and of approximately the same size), or complex/irregular (when the lobules were asymmetric or multiple lobules were seen).
Statistical analysis
Statistical analysis was performed using Stata/IC 14.2 for Windows (StataCorp LP, 4905 Lakeway Drive, College Station, TX 77845, USA). Numeric variables were presented in the form of mean ± SD (min − max) and categorical variables as frequencies. Correlative analyses were performed using the Mann–Whitney U test, Fisher's exact test, or the Chi-square test. P values less than 0.05 were considered statistically significant.
RESULTS
During the study period, a total of 694 patients with aneurysmal SAH were admitted to our institution. We identified 113 patients who met our inclusion and exclusion criteria. The majority of the patients were female (n = 84, 74.3%). The average age of the patients was 51.7 ± 12.3 years (range 21.8–85.6 years).
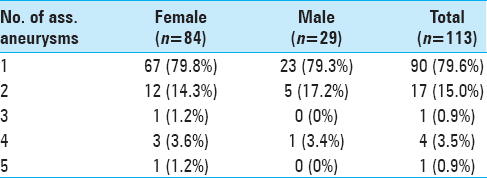
The majority of the patients had only one associate unruptured aneurysm (79.6%), but up to 5 associated unruptured aneurysms were recorded. The total number of associated unruptured aneurysms was 148. The number of associated aneurysms is shown in
Aneurysmal size, location, and morphology
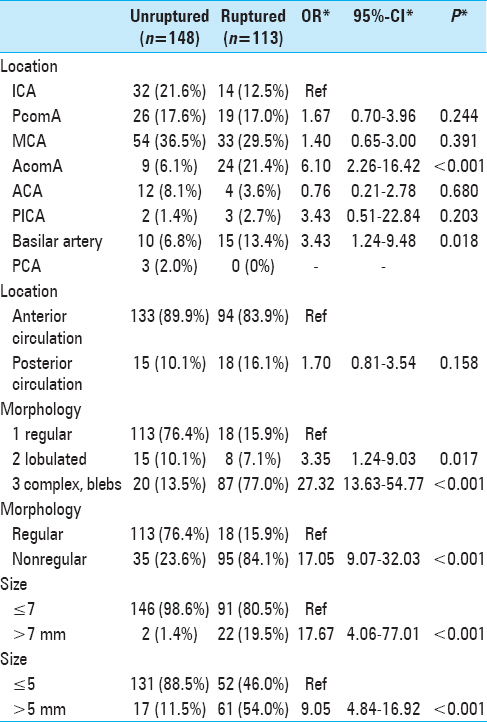
The average size of the unruptured aneurysms was 3.1 ± 1.5 mm (range 1.0–9.5 mm), and the average size of the ruptured aneurysms was 5.7 ± 2.7 mm (range 1.8–19.0 mm). There was a significant difference in the size of ruptured and unruptured aneurysms (P < 0.001). The unruptured aneurysms were overwhelmingly <7 mm (n = 146, 98.6%), with the majority being <5 mm (n = 131, 88.5%). Similarly, the majority of the ruptured aneurysms were also <7 mm (n = 91, 80.5%) and close to half were smaller than 5 mm (n = 52, 46.0%). The AR was significantly larger in ruptured aneurysms (1.8 ± 0.7, range 0.6–3.8 vs. 1.5 ± 0.5 range 0.4–2.9, P < 0.001) between the two cohorts. Similarly, the bottleneck factor was also significantly larger in ruptured aneurysms (1.7 ± 0.6, range 0.7–3.8 vs. 1.3 ± 0.4, range 0.1–3.0, P < 0.001).
The majority of the aneurysms were located in the anterior circulation (n = 227, 86.9%) with the majority of the ruptured aneurysms located in the anterior circulation (n = 94, 83.9%). The most frequent location for both the unruptured and ruptured aneurysms was the MCA (n = 54, 36.0% and n = 33, 29.5%, respectively), with the ICA representing the second most frequent location of unruptured aneurysms and the AcomA representing the second most frequent location for ruptured aneurysms.
The majority of the unruptured aneurysms were smooth and had a regular morphology (n = 113, 76.4%) compared to only 15.9% of ruptured aneurysms. Conversely, a significant majority of ruptured aneurysms were irregular (n = 87, 77.0%) with a minority of unruptured aneurysms having an irregular morphology (n = 20, 13.5%). The aneurysm characteristics are shown in
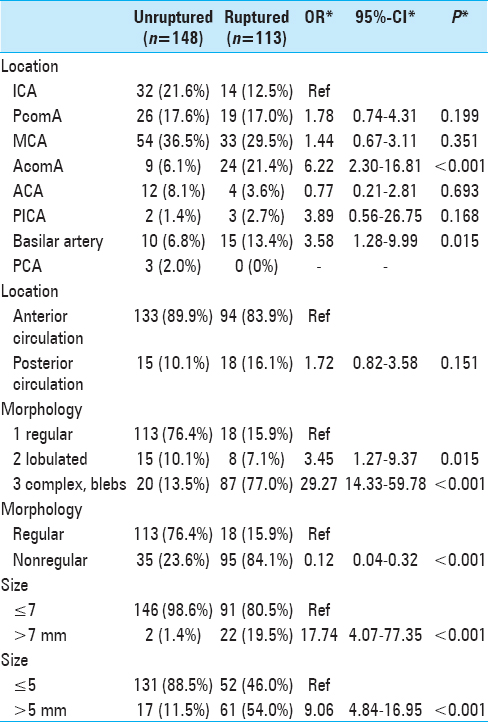
In the multivariate analysis and after matching for age and sex, aneurysm location, aneurysm morphology, and size were independently associated with rupture. A complex aneurysm morphology was the strongest risk factor for rupture (OR, 29.27; 95% CI, 14.33–59.78; P < 0.001) with size >7 mm (OR, 17.74; 95% CI, 4.07–77.35; P < 0.001) and AcomA location also showing a strong independent association. Interestingly, although a lobulated appearance showed an increased risk, this did not reach statistical significance (OR, 3.45; 95% CI, 1.27–9.37; P < 0-001 0.015), suggesting that an increasingly irregular morphology is important in the risk of rupture. The characteristics of the two cohorts after matching for age and sex are shown in
DISCUSSION
A variety of factors have been implicated in the rupture of aneurysms. Aneurysmal dome size is naturally one of the most widely recognized risk factors, and at the moment size still remains the most widely used geometrical factor when determining the decision to treat. Aneurysms over 10 mm carry a 1.9% per year risk of rupture; however, size alone cannot be used as the only measure upon which to base treatment decisions, and recent studies have shown that size alone is not a good predictor.[
The size ratio, introduced by Dhar et al.,[
Irregular shape is another important variable linked to rupture. A Japanese observational study demonstrated that unruptured aneurysms with daughter sacs have a higher rupture rate than aneurysms with regular shapes,[
Applying the results of post-rupture morphological analysis to determine the risk of rupture relies on the premise that post-rupture morphology is not significantly altered from the pre-rupture state.[
Routine imaging with MRI or CT angiography is the accepted standard of care for patients with small aneurysms with treatment considered if there are signs of growth. There is an increasing awareness that imaging of the wall may help to delineate which aneurysms are at risk of growth and rupture prior to any actual morphological change. One technique that shows early promise is contrast-enhanced black blood vessel wall MRI. Several cases and case series have been published that have shown a high accuracy in determining which aneurysm has ruptured in cases of multiple aneurysms.[
Our study is limited by the inherent weaknesses of a retrospective study design and the fact that visual confirmation of the ruptured aneurysm was not obtained in all aneurysms. A further limitation is that the measured variables such as irregularity and size may be a result of aneurysm rupture rather than a cause. We believe that future prospective studies should include aneurysm wall imaging preferably with black blood contrast-enhanced MRI. These studies may help to highlight the potential to detect inflammation and wall changes prior to rupture in aneurysms irrespective of size, location, etc., and help to risk stratify patients appropriately.
CONCLUSION
Aneurysm size is important in determining the risk of rupture; however, the morphology of aneurysms is also extremely important, and as others have reported the shape of an aneurysm may be more important in determining its rupture risk. We believe that rather than relying on size alone we must consider a larger number of features when determining the treatment strategy for our patients. As has been shown the pre and post-rupture appearance cannot be assumed to be the same, and a pre-rupture evaluation of the aneurysm wall is essential in accurately risk stratifying patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abboud T, Rustom J, Bester M, Czorlich P, Vittorazzi E, Pinnschmidt HO. Morphology of Ruptured and Unruptured Intracranial Aneurysms. World Neurosurg. 2017. 99: 610-7
2. Amenta PS, Yadla S, Campbell PG, Maltenfort MG, Dey S, Ghosh S. Analysis of nonmodifiable risk factors for intracranial aneurysm rupture in a large, retrospective cohort. Neurosurgery. 2012. 70: 693-701
3. Backes D, Vergouwen MDI, Velthuis BK, van der Schaaf IC, Bor ASE, Algra A. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke J Cereb Circ. 2014. 45: 1299-303
4. Baharoglu MI, Lauric A, Gao B-L, Malek AM. Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg. 2012. 116: 871-81
5. Beck J, Rohde S, el Beltagy M, Zimmermann M, Berkefeld J, Seifert V. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir (Wien). 2003. 145: 861-5
6. Bhogal P, Navaei E, Makalanda HLD, Brouwer PA, Sjöstrand C, Mandell DM. Intracranial vessel wall MRI. Clin Radiol. 2016. 71: 293-303
7. Bhogal P, Uff C, Makalanda HLD. Vessel wall MRI and intracranial aneurysms. J Neurointervent Surg. 2016. 8: 1160-2
8. Björkman J, Frösen J, Tähtinen O, Backes D, Huttunen T, Harju J. Irregular Shape Identifies Ruptured Intracranial Aneurysm in Subarachnoid Hemorrhage Patients With Multiple Aneurysms. Stroke. 2017. 48: 1986-9
9. Bor ASE, Tiel Groenestege AT, terBrugge KG, Agid R, Velthuis BK, Rinkel GJE. Clinical, radiological, and flow-related risk factors for growth of untreated, unruptured intracranial aneurysms. Stroke. 2015. 46: 42-8
10. Brinjikji W, Zhu Y-Q, Lanzino G, Cloft HJ, Murad MH, Wang Z. Risk Factors for Growth of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2016. 37: 615-20
11. Brown RD, Broderick JP. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014. 13: 393-404
12. Chmayssani M, Rebeiz JG, Rebeiz TJ, Batjer HH, Bendok BR. Relationship of growth to aneurysm rupture in asymptomatic aneurysms ≤7 mm: A systematic analysis of the literature. Neurosurgery. 2011. 68: 1164-171
13. Choi JH, Jo KI, Kim KH, Jeon P, Yeon JY, Kim JS. Morphological risk factors for the rupture of anterior communicating artery aneurysms: The significance of fenestration. Neuroradiology. 2016. 58: 155-60
14. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008. 63: 185-97
15. Edjlali M, Gentric J-C, Régent-Rodriguez C, Trystram D, Hassen WB, Lion S. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms?. Stroke J Cereb Circ. 2014. 45: 3704-6
16. Forget TR, Benitez R, Veznedaroglu E, Sharan A, Mitchell W, Silva M. A review of size and location of ruptured intracranial aneurysms. Neurosurgery. 2001. 49: 1322-6
17. Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
18. Harada K, Fukuyama K, Shirouzu T, Ichinose M, Fujimura H, Kakumoto K. Prevalence of unruptured intracranial aneurysms in healthy asymptomatic Japanese adults: Differences in gender and age. Acta Neurochir (Wien). 2013. 155: 2037-43
19. Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA. Early Change in Ferumoxytol-Enhanced Magnetic Resonance Imaging Signal Suggests Unstable Human Cerebral Aneurysm.A Pilot Study. Stroke J Cereb Circ. 2012. 43: 3258-65
20. Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH. Bottleneck factor and height-width ratio: Association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007. 61: 716-23
21. Hu P, Yang Q, Wang D-D, Guan S-C, Zhang H-Q. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology. 2016. 58: 979-85
22. Hu P, Yang Q, Wang D-D, Guan S-C, Zhang H-Q. Wall enhancement on high-resolution magnetic resonance imaging may predict an unsteady state of an intracranial saccular aneurysm. Neuroradiology. 2016. 58: 979-85
23. Huang Z-Q, Meng Z-H, Hou Z-J, Huang S-Q, Chen J-N, Yu H. Geometric Parameter Analysis of Ruptured and Unruptured Aneurysms in Patients with Symmetric Bilateral Intracranial Aneurysms: A Multicenter CT Angiography Study. AJNR Am J Neuroradiol. 2016. 37: 1413-7
24. Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H. Unruptured intracranial aneurysms: Incidence of rupture and risk factors. Stroke J Cereb Circ. 2009. 40: 313-6
25. Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J Neurosurg. 2000. 93: 379-87
26. Kim MC, Hwang S-K. The Rupture Risk of Aneurysm in the Anterior Communicating Artery: A Single Center Study. J Cerebrovasc Endovasc Neurosurg. 2017. 19: 36-43
27. Kondo R, Yamaki T, Mouri W, Sato S, Saito S, Nagahata M. [Magnetic resonance vessel wall imaging reveals rupture site in subarachnoid hemorrhage with multiple cerebral aneurysms]. No Shinkei Geka. 2014. 42: 1147-50
28. Kono K, Tomura N, Yoshimura R, Terada T. Changes in wall shear stress magnitude after aneurysm rupture. Acta Neurochir (Wien). 2013. 155: 1559-63
29. Lauric A, Baharoglu MI, Malek AM. Ruptured status discrimination performance of aspect ratio, height/width, and bottleneck factor is highly dependent on aneurysm sizing methodology. Neurosurgery. 2012. 71: 38-45
30. Lin N, Ho A, Charoenvimolphan N, Frerichs KU, Day AL, Du R. Analysis of morphological parameters to differentiate rupture status in anterior communicating artery aneurysms. PloS One. 2013. 8: e79635-
31. Lin N, Ho A, Gross BA, Pieper S, Frerichs KU, Day AL. Differences in simple morphological variables in ruptured and unruptured middle cerebral artery aneurysms. J Neurosurg. 2012. 117: 913-9
32. Lindgren AE, Koivisto T, Björkman J, von Und Zu Fraunberg M, Helin K, Jääskeläinen JE. Irregular Shape of Intracranial Aneurysm Indicates Rupture Risk Irrespective of Size in a Population-Based Cohort. Stroke J Cereb Circ. 2016. 47: 1219-26
33. Liu J, Xiang J, Zhang Y, Wang Y, Li H, Meng H. Morphologic and hemodynamic analysis of paraclinoid aneurysms: Ruptured versus unruptured. J Neurointerventional Surg. 2014. 6: 658-63
34. Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery. 2013. 72: 492-6
35. Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y. Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J. Neurosurg. 2014. 120: 104-10
36. Menghini VV, Brown RD, Sicks JD, O’Fallon WM, Wiebers DO. Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted County, Minnesota, 1965 to 1995. Neurology. 1998. 51: 405-11
37. Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND. Is aspect ratio a reliable predictor of intracranial aneurysm rupture?. Neurosurgery. 2004. 54: 1343-8
38. Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S. Wall Enhancement of the Intracranial Aneurysms Revealed by Magnetic Resonance Vessel Wall Imaging Using Three-Dimensional Turbo Spin-Echo Sequence with Motion-Sensitized Driven-Equilibrium: A Sign of Ruptured Aneurysm?. Clin Neuroradiol. 2016. 26: 277-83
39. Nakagawa T, Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg. 1994. 80: 217-23
40. Omodaka S, Endo H, Niizuma K, Fujimura M, Endo T, Sato K. Circumferential Wall Enhancement on Magnetic Resonance Imaging is Useful to Identify Rupture Site in Patients with Multiple Cerebral Aneurysms. Neurosurgery. 2017. p.
41. Qiu T, Jin G, Xing H, Lu H. Association between hemodynamics, morphology, and rupture risk of intracranial aneurysms: A computational fluid modeling study. Neurol Sci. 2017. 38: 1009-18
42. Raghavan ML, Ma B, Harbaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg. 2005. 102: 355-62
43. Rahman M, Ogilvy CS, Zipfel GJ, Derdeyn CP, Siddiqui AH, Bulsara KR. Unruptured cerebral aneurysms do not shrink when they rupture: Multicenter collaborative aneurysm study group. Neurosurgery. 2011. 68: 155-61
44. Rahman M, Smietana J, Hauck E, Hoh B, Hopkins N, Siddiqui A. Size ratio correlates with intracranial aneurysm rupture status: A prospective study. Stroke. 2010. 41: 916-20
45. de Rooij NK, Velthuis BK, Algra A, Rinkel GJE. Configuration of the circle of Willis, direction of flow, and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J Neurol. 2009. 256: 45-50
46. Ryu C-W, Kwon O-K, Koh JS, Kim EJ. Analysis of aneurysm rupture in relation to the geometric indices: Aspect ratio, volume, and volume-to-neck ratio. Neuroradiology. 2011. 53: 883-9
47. Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008. 62: 602-9
48. Schneiders JJ, Marquering HA, van den Berg R, VanBavel E, Velthuis B, Rinkel GJE. Rupture-associated changes of cerebral aneurysm geometry: High-resolution 3D imaging before and after rupture. AJNR Am J Neuroradiol. 2014. 35: 1358-62
49. Skodvin TØ, Johnsen L-H, Gjertsen Ø, Isaksen JG, Sorteberg A. Cerebral Aneurysm Morphology Before and After Rupture: Nationwide Case Series of 29 Aneurysms. Stroke. 2017. 48: 880-6
50. Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010. 41: 1969-77
51. Tateshima S, Chien A, Sayre J, Cebral J, Viñuela F. The effect of aneurysm geometry on the intra-aneurysmal flow condition. Neuroradiology. 2010. 52: 1135-41
52. Teo M, St George EJ. Radiologic Surveillance of Untreated Unruptured Intracranial Aneurysms: A Single Surgeon's Experience. World Neurosurg. 2016. 90: 20-8
53. Tremmel M, Dhar S, Levy EI, Mocco J, Meng H. Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: Results from a virtual experimental study. Neurosurgery. 2009. 64: 622-31
54. UCAS Japan Investigators, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
55. Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm?. Neurosurgery. 2001. 48: 495-503
56. . Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998. p. 1725-33
57. Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: Growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013. 269: 258-65
58. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
59. Wang GX, Zhang D, Wang ZP, Yang LQ, Zhang L, Wen L. Risk Factors for the Rupture of Bifurcation Intracranial Aneurysms Using CT Angiography. Yonsei Med J. 2016. 57: 1178-84
60. Weir B, Amidei C, Kongable G, Findlay JM, Kassell NF, Kelly J. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003. 99: 447-51
61. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD, Piepgras DG. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet Lond Engl. 2003. 362: 103-10
62. Xiang J, Yu J, Choi H, Dolan Fox JM, Snyder KV, Levy EI. Rupture Resemblance Score (RRS): Toward risk stratification of unruptured intracranial aneurysms using hemodynamic-morphological discriminants. J Neurointervent Surg. 2015. 7: 490-5
63. Xu T, Lin B, Liu S, Shao X, Xia N, Zhang Y. Larger size ratio associated with the rupture of very small (≤3 mm) anterior communicating artery aneurysms. J Neurointervent Surg. 2017. 9: 278-2
64. Yi J, Zielinski D, Chen M. Cerebral Aneurysm Size before and after Rupture: Case Series and Literature Review. J Stroke Cerebrovasc Dis. 2016. 25: 1244-8
65. Zanaty M, Chalouhi N, Tjoumakaris SI, Fernando Gonzalez L, Rosenwasser RH, Jabbour PM. Aneurysm geometry in predicting the risk of rupture. A review of the literature. Neurol Res. 2014. 36: 308-13
66. Zhang Y, Mu S, Chen J, Wang S, Li H, Yu H. Hemodynamic analysis of intracranial aneurysms with daughter blebs. Eur Neurol. 2011. 66: 359-67