- Department of Neurosurgery, Desert Regional Medical Center, Palm Springs, CA,
- College of Osteopathic Medicine, University of New England, Biddeford, ME,
- College of Medicine, University of Kentucky, Lexington, KY,
- School of Medicine, University of Texas Medical Branch, Galveston, TX,
- Pre-Medical Studies, Chapman University, Orange, CA,
- College of Literature, Arts, and Sciences, University of Michigan-Flint, Flint, MI, United States,
- College of Osteopathic Medicine, Michigan State University, East Lansing,
- Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, United States.
Correspondence Address:
Brian Fiani
Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor, MI, United States.
DOI:10.25259/SNI_495_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Brian Fiani1, Christian Noblett2, Jacob Nanney3, Thao Doan4, Elisabeth Pennington5, Ryan Jarrah6, Erika Sarno7, Daniel Nikolaidis8. Diffusion tensor imaging of the spinal cord status post trauma. 05-Sep-2020;11:276
How to cite this URL: Brian Fiani1, Christian Noblett2, Jacob Nanney3, Thao Doan4, Elisabeth Pennington5, Ryan Jarrah6, Erika Sarno7, Daniel Nikolaidis8. Diffusion tensor imaging of the spinal cord status post trauma. 05-Sep-2020;11:276. Available from: https://surgicalneurologyint.com/surgicalint-articles/10241/
Abstract
Background: Since its development in 1994, diffusion tensor imaging (DTI) has been successfully used to assess structural and functional changes to neurological tissue within the central nervous system. Namely, DTI is a noninvasive magnetic resonance imaging (MRI)-based technique that uses anisotropic diffusion to visualize and estimate the organization of white matter in neuronal tissue. It has been used to study various spinal pathologies including neoplastic diseases, degenerative myelopathy, demyelinating diseases, and infections involving the spinal cord. However, due to technical uncertainties and experimental limitations, DTI has rarely been clinically applied to assess trauma-related spinal pathologies.
Methods: An extensive review of the published literature on DTI was performed utilizing PubMed, OVID Medline, and EMBASE journals. Terms used for the search included DTI and spine trauma.
Results: The search yielded full text English language-related articles regarding DTIs application, limitations, and functional outcomes secondary to spinal trauma.
Conclusion: DTI relies on anisotropy in CNS tissues to determine the spatial orientation of surrounding axon tracts and define anatomical boundaries. Diffusion along three principle axes is used to calculate the following four DTI indices; fractional anisotropy, apparent diffusion coefficient (ADC), longitudinal ADC, and transverse ADC. Using DTI as a diagnostic tool status, post spine trauma has proven useful in examining the morphological and physiological extent of spinal lesions beyond conventional MRI. Experimental studies are now utilizing DTI to analyze the severity of spinal cord trauma during the hyperacute phase and may potentially be used to providing additional diagnostic information for improved treatment efficiency (e.g., as shown during the stem cell therapy trials).
Keywords: Diffusion tensor imaging, Neuroradiology, Neurotrauma, Spinal cord, Trauma
INTRODUCTION
Traumatic spinal cord injury (SCI) can disrupt axonal connectivity.[
DTI has become increasingly incorporated into routine clinical protocols for assessing abnormalities of the brain, while protocols for clinical application to spinal cord pathology are limited. Nevertheless, various animal-based experimental studies found correlations between diffusion tensor measures after SCI and locomotor outcomes, injury severity, white matter tract disruption, and glial scar orientation.[
TECHNOLOGY
The technology of DTI is an elegant collaboration between MRI sequences, with software that utilize the diffusion of water to generate contrast in neural tract images. This technology is derived from diffusion-weighted imaging (DWI), an MRI variant that measures signal strength based on the mean displacement of water in tissues. Today, DTI is now considered more advantageous than DWI because it can also quantify the direction of diffusion.[
PRINCIPLES
Diffusion MRI relies on the intrinsic properties of highly structured tissues, specifically neurons and white matter tracts in the central nervous system. In these tissues, diffusion of water molecules occurs preferentially along one direction.[
FA is a scalar number between 0 and 1 that indicates the degree of anisotropy of molecular water diffusion along and across the axon. Injuries to the spinal cord result in disruption of longitudinally arranged axons, and therefore, DTI will reveal a FA value less than 1.[
Advantages
DTI has many advantages to its use in the clinical setting when examining and diagnosing SCIs compared to plain MRI. Bihan et al. reported that DTI as a quantitative method reflects the properties of tissues while being able to be compared between patients or be compared overtime without a need for standardization.[
Further, Patel et al. continued to discuss that DTI is more sensitive to SCI severity classification than other imaging modalities.[
All in all, DTI is both a sensitive and specific test for SCI within the white matter.[
Disadvantages
DTI appearance is based on the premise that the direction of the fastest diffusion indicates the direction of the fibers as reported by Bihan et al.[
DTI is a difficult technique to analyze and implement on the spinal cord. The artifacts created by the imaging overlay the smaller size of the cord, as well, the physiologic motion created by the heart and lungs distort the image further.[
By limiting the time required to take the image, physiological motion can be reduced to minimal interference. Single shot echo planar imaging is one such modality that can reduce these motion artifacts and distortions to make the image easier to interpret.[
TRIALS AND OUTCOMES
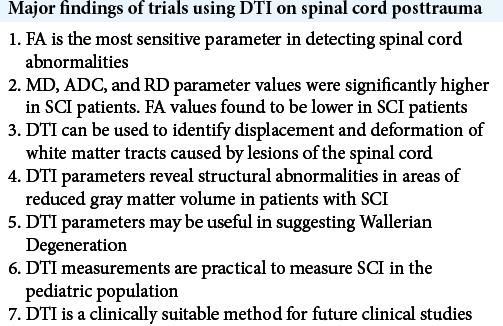
Due to limitations in finding randomized control trials and studies about DTI application secondary to spinal trauma, multiple case reports and clinical studies have investigated the posttraumatic use of DTI on the spinal cord. Studies of DTI of the spinal cord status posttrauma characterize spinal lesions and associated findings using different DTI parameters. The four major DTI parameters investigated in these studies were FA, MD, ADC, and RD. FA is the most frequently referenced DTI metric. Early studies of DTI parameters found that FA was the most sensitive parameter in detecting spinal cord abnormalities [
Available literature suggests that DTI has a variety of clinical applications. DTI parameters may be useful in detecting Wallerian degeneration (WD).[
Using DTI as a diagnostic tool has shown great promise. Several clinical studies have been conducted with findings that support its use in the clinical setting. Multiple DTI parameters have proven useful in examining the morphological and physiological extent of spinal lesions beyond conventional MRI capabilities. DTI parameters have also been shown to correlate with clinical findings. A review of available literature reveals an absence of more recent clinical studies and a lack of randomized clinical control trials further investigating DTI in spine trauma. Larger trials are necessary to further evaluate DTI of the spinal cord posttrauma before mainstream clinical use.
FUTURE CONSIDERATIONS
The future usage of DTI technology in analyzing posttraumatic spinal pathologies is being further trialed for clinical significance. Experimental studies are now utilizing DTI to analyze the severity of spinal cord trauma during the hyperacute phase (first 6 h posttrauma).[
CONCLUSION
Studies are needed to better characterize variations in DTI parameters in various tissue pathologies such as scar formation due to astrocyte aggregation, cerebrospinal fluid infiltration, demyelination, hemorrhage, and edema.[
In conclusion, the usage of DTI to detect and evaluate spinal pathologies related to trauma has shown great promise as a conventional diagnostic technique and will continue to develop as a neuropathological biomarker.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007. 4: 316-29
2. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994. 66: 259-67
3. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995. 8: 333-44
4. Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994. 31: 394-400
5. Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A. Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol. 2011. 21: 426-33
6. Chang Y, Jung TD, Yoo DS, Hyun JK. Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma. 2010. 27: 2033-40
7. Cheran S, Shanmuganathan K, Zhuo J, Mirvis SE, Aarabi B, Alexander MT. Correlation of MR diffusion tensor imaging parameters with ASIA motor scores in hemorrhagic and nonhemorrhagic acute spinal cord injury. J Neurotrauma. 2011. 28: 1881-92
8. D’Souza MM, Choudhary A, Poonia M, Kumar P, Khushu S. Diffusion tensor MR imaging in spinal cord injury. Injury. 2017. 48: 880-4
9. Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD. Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol. 2008. 29: 1976-82
10. Endo T, Suzuki S, Utsunomiya A, Uenohara H, Tominaga T. Prediction of neurological recovery using apparent diffusion coefficient in cases of incomplete spinal cord injury. Neurosurgery. 2011. 68: 329-36
11. Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D. MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol. 2005. 26: 1587-94
12. Freund P, Schneider T, Nagy Z, Hutton C, Weiskopf N, Friston K. Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS One. 2012. 7: e51729
13. Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M. Retrograde wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res. 2008. 86: 2271-80
14. Huisman TA, Loenneker T, Barta G, Bellemann ME, Hennig J, Fischer JE. Quantitative diffusion tensor MR imaging of the brain: Field strength related variance of apparent diffusion coefficient (ADC) and fractional anisotropy (FA) scalars. Eur Radiol. 2006. 16: 1651-8
15. Kamble RB, Venkataramana NK, Naik AL, Rao SV. Diffusion tensor imaging in spinal cord injury. Indian J Radiol Imaging. 2011. 21: 221-4
16. Kelley BJ, Harel NY, Kim CY, Papademetris X, Coman D, Wang X. Diffusion tensor imaging as a predictor of locomotor function after experimental spinal cord injury and recovery. J Neurotrauma. 2014. 31: 1362-73
17. Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, Trinkaus K. Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma. 2010. 27: 587-98
18. Kim JH, Song SK, Burke DA, Magnuson DS. Comprehensive locomotor outcomes correlate to hyperacute diffusion tensor measures after spinal cord injury in the adult rat. Exp Neurol. 2012. 235: 188-96
19. Koerte I, Muehlmann M, Mulert C, Shenton M.editors. Diffusion tensor imaging. MRI in Psychiatry. Berlin, Heidelberg: Springer; 2014. p.
20. Koskinen E, Brander A, Hakulinen U, Luoto T, Helminen M, Ylinen A. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma. 2013. 30: 1587-95
21. Koskinen EA, Hakulinen U, Brander AE, Luoto TM, Ylinen A, Ohman JE. Clinical correlates of cerebral diffusion tensor imaging findings in chronic traumatic spinal cord injury. Spinal Cord. 2014. 52: 202-8
22. Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001. 13: 534-46
23. Lee S, Lee YH, Chung TS, Jeong EK, Kim S, Yoo YH. Accuracy of diffusion tensor imaging for diagnosing cervical spondylotic myelopathy in patients showing spinal cord compression. Korean J Radiol. 2015. 16: 1303-12
24. Li DC, Malcolm JG, Rindler RS, Baum GR, Rao A, Khurpad SN. The role of diffusion tensor imaging in spinal pathology: A review. Neurol India. 2017. 65: 982-92
25. Loy DN, Kim JH, Xie M, Schmidt RE, Trinkaus K, Song SK. Diffusion tensor imaging predicts hyperacute spinal cord injury severity. J Neurotrauma. 2007. 24: 979-90
26. Maki S, Koda M, Saito J, Takahashi S, Inada T, Kamiya K. Tract-specific diffusion tensor imaging reveals laterality of neurological symptoms in patients with cervical compression myelopathy. World Neurosurg. 2016. 96: 184-90
27. Martin AR, de Leener B, Cohen-Adad J, Cadotte DW, Kalsi-Ryan S, Lange SF. Clinically feasible microstructural MRI to quantify cervical spinal cord tissue injury using DTI, MT, and T2*-weighted imaging: Assessment of normative data and reliability. AJNR Am J Neuroradiol. 2017. 38: 1257-65
28. Mohamed FB, Hunter LN, Barakat N, Liu CS, Sair H, Samdani AF. Diffusion tensor imaging of the pediatric spinal cord at 1.5T: Preliminary results. AJNR Am J Neuroradiol. 2011. 32: 339-45
29. O’Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am. 2011. 22: 185-96
30. Patel SP, Smith TD, VanRooyen JL, Powell D, Cox DH, Sullivan PG. Serial diffusion tensor imaging in vivo predicts long-term functional recovery and histopathology in rats following different severities of spinal cord injury. J Neurotrauma. 2016. 33: 917-28
31. Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012. 29: 1556-66
32. Rajasekaran S, Kanna RM, Karunanithi R, Shetty AP. Diffusion tensor tractography demonstration of partially injured spinal cord tracts in a patient with posttraumatic brown sequard syndrome. J Magn Reson Imaging. 2010. 32: 978-81
33. Schwartz ED, Duda J, Shumsky JS, Cooper ET, Gee J. Spinal cord diffusion tensor imaging and fiber tracking can identify white matter tract disruption and glial scar orientation following lateral funiculotomy. J Neurotrauma. 2005. 22: 1388-98
34. Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008. 29: 655-9
35. Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013. 7: 31
36. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003. 20: 1714-22
37. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002. 17: 1429-36
38. Tay B, Hyun JK, Oh S. A machine learning approach for specification of spinal cord injuries using fractional anisotropy values obtained from diffusion tensor images. Comput Math Methods Med. 2014. 2014: 276589
39. Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011. 65: 1532-56
40. Tsuchiya K, Fujikawa A, Honya K, Nitatori T, Suzuki Y. Diffusion tensor tractography of the lower spinal cord. Neuroradiology. 2007. 50: 221-5
41. Vargas MI, Delavelle J, Jlassi H, Rilliet B, Viallon M, Becker CD. Clinical applications of diffusion tensor tractography of the spinal cord. Neuroradiology. 2008. 50: 25-9
42. Vedantam A, Eckardt G, Wang MC, Schmit BD, Kurpad SN. Clinical correlates of high cervical fractional anisotropy in acute cervical spinal cord injury. World Neurosurg. 2015. 83: 824-8
43. Vedantam A, Jirjis MB, Schmit BD, Budde MD, Ulmer JL, Wang MC. Diffusion tensor imaging and tractography in Brown-Sequard syndrome. Spinal Cord. 2012. 50: 928-30
44. Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Diffusion tensor imaging of the spinal cord: Insights from animal and human studies. Neurosurgery. 2014. 74: 1-8
45. Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009. 19: 224-32
46. Zaninovich OA, Avila MJ, Kay M, Becker JL, Hurlbert RJ, Martirosyan NL. The role of diffusion tensor imaging in the diagnosis, prognosis, and assessment of recovery and treatment of spinal cord injury: A systematic review. Neurosurg Focus. 2019. 46: E7
47. Zhao C, Rao JS, Pei XJ, Lei JF, Wang ZJ, Zhao W. Diffusion tensor imaging of spinal cord parenchyma lesion in rat with chronic spinal cord injury. Magn Reson Imaging. 2018. 47: 25-32