- Department of Neurology and Neurosurgery, McGill University, Montreal, Quebec, Canada,
- Department of Neurosurgery, Ibn Sina Hospital, Ministry of Health, Kuwait City, Kuwait,
- Kuwait Medical School, Health Sciences Center, Kuwait University, Jabriya, Kuwait,
- Department of Medicine, Toronto University, Toronto, Ontario, Canada,
- Department of Neurosciences and Reproductive and Odontostomatological Sciences, Division of Neurosurgery, University of Napoli Federico II, Naples, Italy.
Correspondence Address:
Dragan Savic, Department of Neurosurgery, Ibn Sina Hospital, Kuwait City, Kuwait.
DOI:10.25259/SNI_914_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmad Kh Alhaj1, Waleed Yousef2, Abdulrahman Alanezi2, Mariam Almutawa3, Salem Zaidan2, Tarik M. Alsheikh2, Moussa Abdulghaffar4, Tariq Al-Saadi1, Luigi M. Cavallo5, Dragan Savic2. Does establishing a neurovascular unit improve the outcome after surgical clipping for aneurysmal subarachnoid hemorrhage? Results from a 5-year observational study in Kuwait. 02-Nov-2021;12:547
How to cite this URL: Ahmad Kh Alhaj1, Waleed Yousef2, Abdulrahman Alanezi2, Mariam Almutawa3, Salem Zaidan2, Tarik M. Alsheikh2, Moussa Abdulghaffar4, Tariq Al-Saadi1, Luigi M. Cavallo5, Dragan Savic2. Does establishing a neurovascular unit improve the outcome after surgical clipping for aneurysmal subarachnoid hemorrhage? Results from a 5-year observational study in Kuwait. 02-Nov-2021;12:547. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11209
Abstract
Background: Failure to prevent rebleeding after cerebral subarachnoid hemorrhage (SAH) is the most frequent reason for high morbidity and mortality of aneurysmal SAH. Our study aims to identify the outcome after surgical clipping of aneurysmal SAH before and after the establishment of the neurovascular unit. The clarifications of the positive turnover in the outcome will be discussed.
Methods: A retrospective cohort analysis was carried out on our experience with a controlled group of patients who underwent clipping for ruptured cerebral aneurysms (n = 61) from January 2015 to December 2019. A modified Rankin scale (mRS) was used to determine the outcome after 6 months of follow-up.
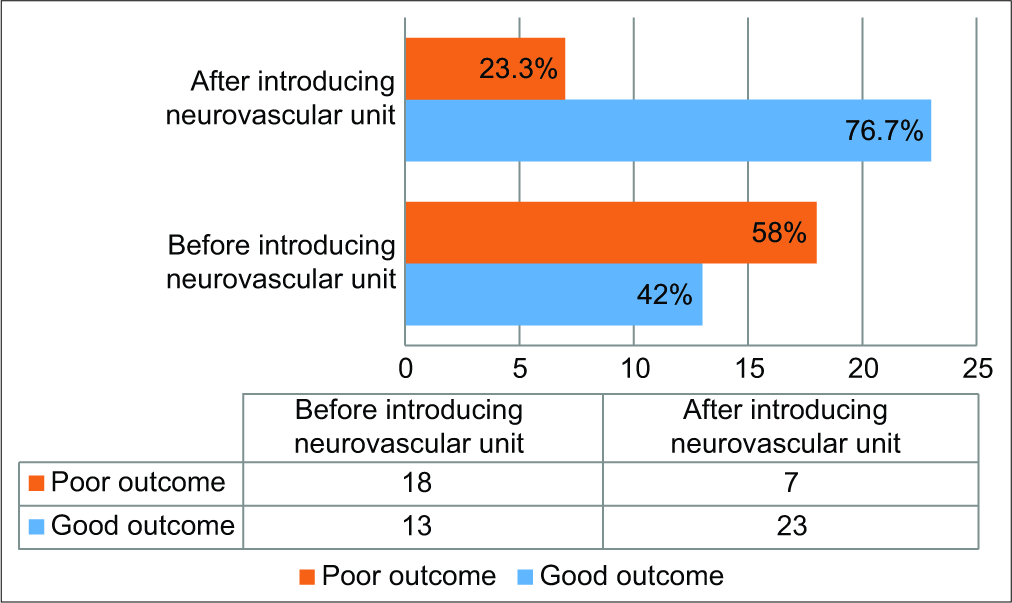
Results: The median mRS score (i.e., outcome) on admission was 4, whereas it was with a median score of 2 six months after clipping (P ≤ 0.001). Overall, the cases with a good outcome were 63.9% of the sample, while the poor outcome conditions were 36.1%. The most cases with an improved outcome were after introducing the neurovascular unit, representing a transition of aneurysmal clipping practice in our center. The good outcome was changed from 42% to 76.7%, and the poor outcome was changed from 58% to 23.3% (P = 0.019). The crude mortality rate was similar to the rate worldwide (18%), with a noticeable decrease after organizing a neurovascular subspecialty.
Conclusion: The outcome after clipping of ruptured SAH can be largely affected by the surgeon’s experience and postoperative intensive care. Organizing a neurovascular team is one of the major factors to achieve good outcomes.
Keywords: Cerebral aneurysm, Clipping, Outcome, Subarachnoid hemorrhage, Surgeon experience
INTRODUCTION
A ruptured cerebral aneurysm would remain a major reason for high morbidity and mortality, especially if there were no early interventions.[
Both clipping and coiling have pros and cons, which makes them complementary to each other rather than competitive.[
Of course, the dominant treatment modality for ruptured cerebral aneurysms is coiling. Thus, coiling as a treatment modality began to emerge in Kuwait in 2014. However, several cases that we faced were not amenable to coiling. Hence, surgical clipping certainly remains a major treatment option for aneurysmal SAH. Almost all the patients presented here underwent a trial of coiling before the surgical clipping. In this analysis, we do not compare the two modalities for resolving ruptured cerebral aneurysms, but we aim to compare the safety, efficacy, and outcome of treating patients with clipping before and after the establishment of the neurovascular unit. This is the first analysis in Kuwait that outlines the experience of surgical clipping of ruptured SAH. Our prespecified hypothesis intents to show that the surgeon’s experience in a coherent center is one of the most important factors affecting patients’ outcome. We also underline the predictors that should be addressed to improve the outcome.
MATERIALS AND METHODS
Study design, setting, and participants
In this retrospective cohort, we enrolled 61 patients from Ibn Sina Hospital in Kuwait from January 2015 until December 2019. We studied all available records for patients who underwent surgical clipping for a cerebral aneurysm in that period. This scheme was used to ensure the representativeness of all cases in Kuwait since it is the only hospital that performs this operation. Our inclusion criterion was any patient who underwent a neurosurgical clipping procedure in our center and was diagnosed with a ruptured aneurysmal SAH that was confirmed before surgery by digital subtraction angiogram except for one case that was operated based on computerized tomography (CT) angiography. Exclusion criteria included clipping performed for nonruptured cerebral aneurysms, cases that underwent endovascular coiling, and SAH cases that were not attributable to a ruptured aneurysm.
The operative theater database of our center was utilized to select the cases in Kuwait. A standardized protocol was followed for data collection to avoid information bias. Each participant with cerebral aneurysmal SAH was followed from the records for 6 months. All our patients after surgical clipping received similar care (protocol for subarachnoid hemorrhage). After the operation, the patient was admitted to the intensive care unit (ICU) for at least 14 days from the onset of the bleeding, regardless of the clinical condition. All patients received nimodipine and antiepileptic, and were followed up daily by a neurointensivist and a neurosurgeon. CT cerebral perfusion was performed to look for vasospasm after the 4th, 6th, and 10th day post-SAH. Afterward, the patients were either shifted to the floor or another medical center for further management and rehabilitation until they were discharged if they were well enough.
Data, variables, and outcome
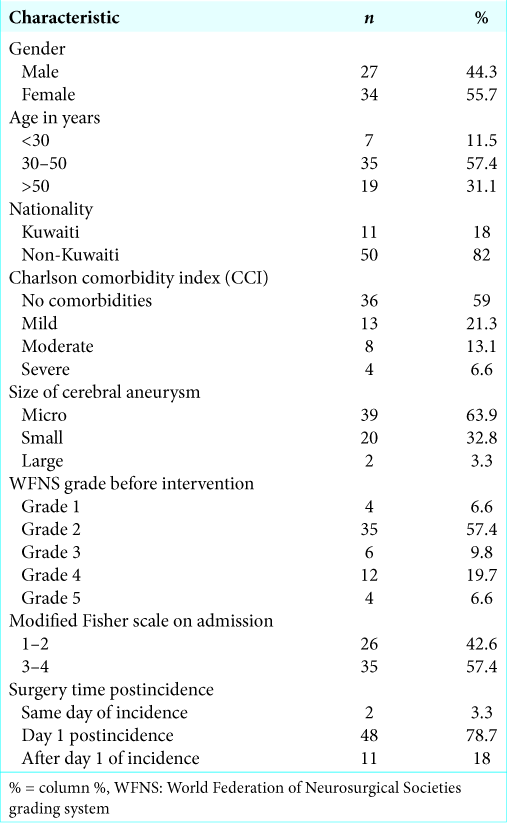
The ethical approval for the conduction of this study was verified by the Standing Committee for Protection of Human Subjects in Research in Kuwait. Patients were viewed back in time by the use of their medical records. These documents included the charts, physician notes, nursing notes, physiotherapist notes, operative notes, ICU notes, radiological images, as well as the system data. We declare that all the information in our paper were derived from patients’ medical file records. A convenient sampling method was used for data collection, and the distribution of qualitative responses was described as frequency and percentages. They were classified based on gender, age (<30 years old, 30–50 years old, or more than 50 years old), and Charlson comorbidity index (CCI)[
Concerning the outcome, the modified Rankin scale (mRS) score during admission and after 6 months was determined for each participant.[
Statistical methods
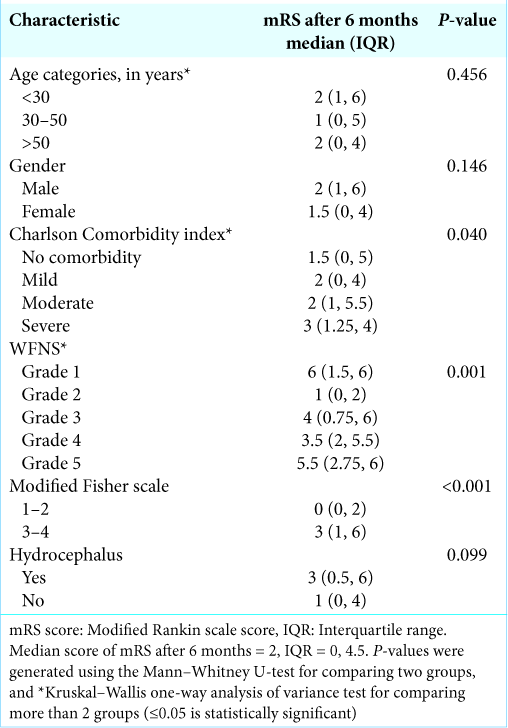
The data were entered and analyzed through IBM Statistical Package for the Social Sciences. The data were inspected for logical data entry errors and cleaned. All qualitative variables were summarized into frequencies and percentages. The non-normally distributed continuous outcome variable (mRS) was summarized with the use of the median and interquartile range (IQR). The difference of medians for the continuous variable was measured to look for any association. Pearson’s Chi-square was used to measure the significance of the associations between the categorical variables. Mann– Whitney U-test was utilized to compare two groups with a non-normal frequency distribution, while Kruskal–Wallis one-way test of variance was used for more than two groups. We tested the hypothesis of our questions in relation to age, gender, CCI, size of the aneurysm, WFNS grade, modified Fisher scale, and development of hydrocephalus and/or vasospasm. P ≤ 0.05 was considered statistically significant.
RESULTS
In our analysis, 66 patients (out of a total of 180 SAH cases) underwent surgical clipping for a ruptured SAH. Out of this number, 61 patients, representing the sample size, were followed for 6 months. Most of the participants (92%) underwent a trial of coiling before the surgical clipping. There were five patients with missing data who were opted out of the study.
In [
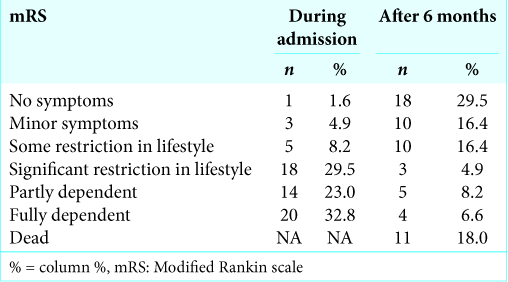
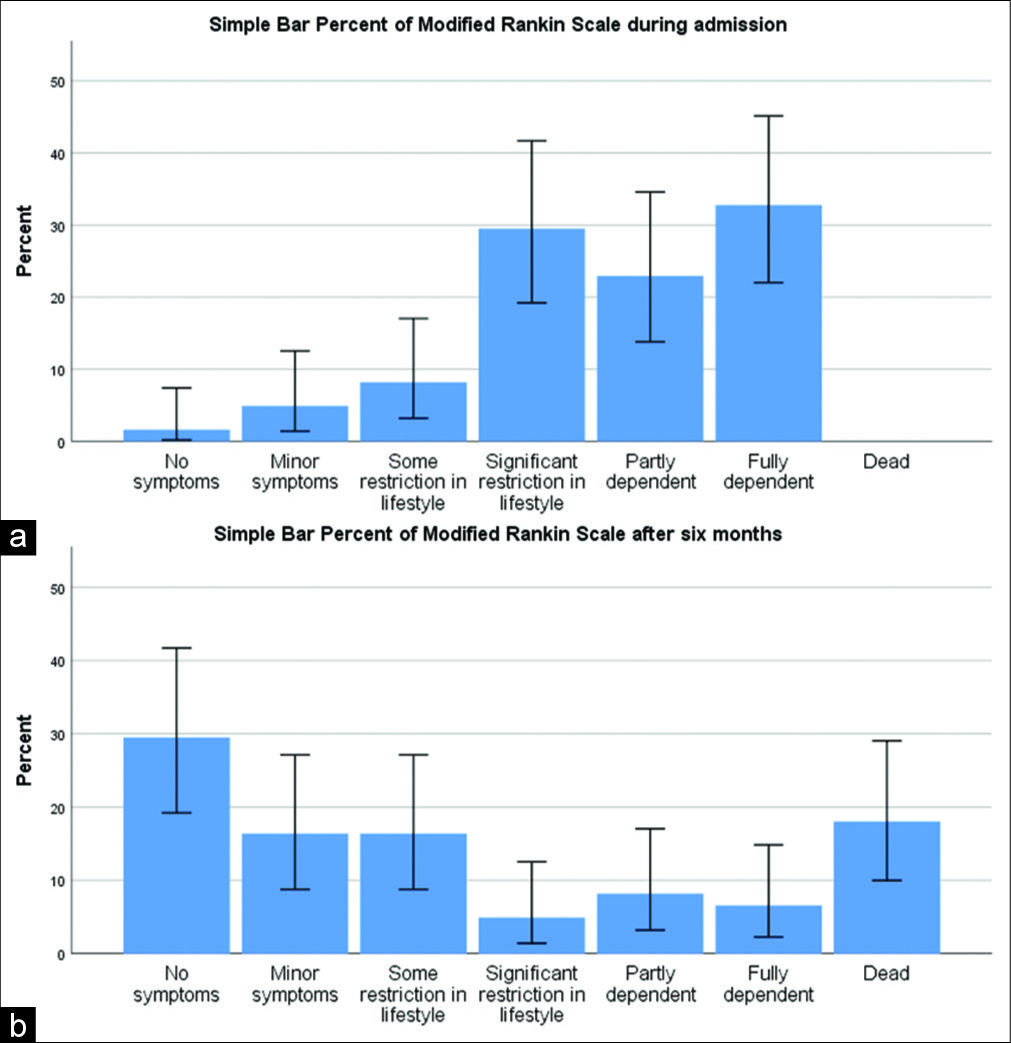
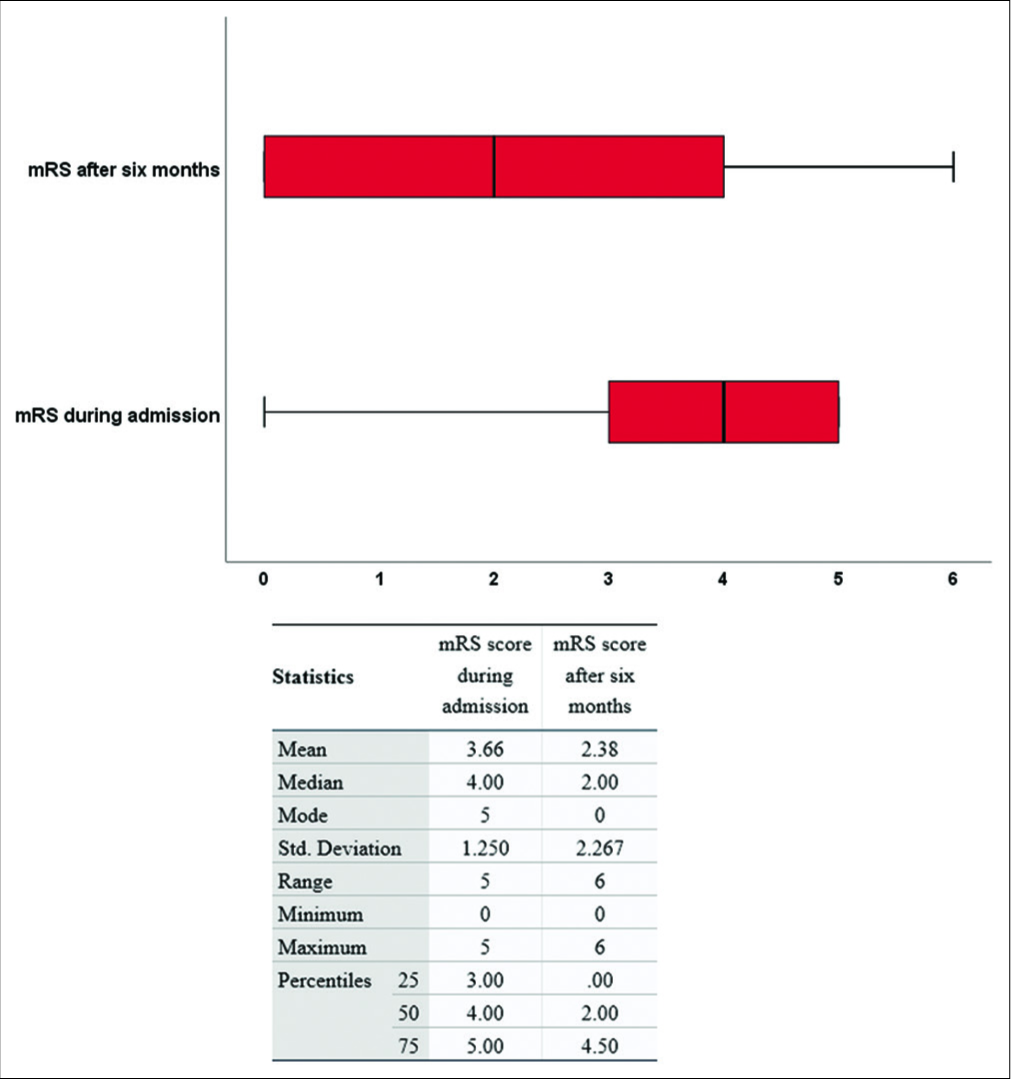
As declared, the median mRS score after 6 months represented our endpoint. The lower the median mRS, the better is the functional outcome. After a 6-month follow-up duration, the good outcome conditions (mRS = 0–2) represented 63.9%, whereas, the poor outcome conditions (mRS = 3–6) represented 36.1%. On hospital admission, the median mRS score of the sample was 4 (IQR: 3–5), while the median mRS score after 6 months was 2 (IQR: 0–4.5). The difference between the medians of mRS was statistically significant (difference = 2, P≤0.001). After 6 months, the outcome was considerably better in comparison to the admission status in a large number of cases [
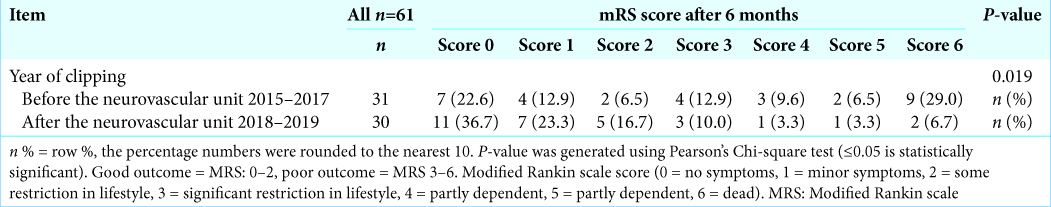
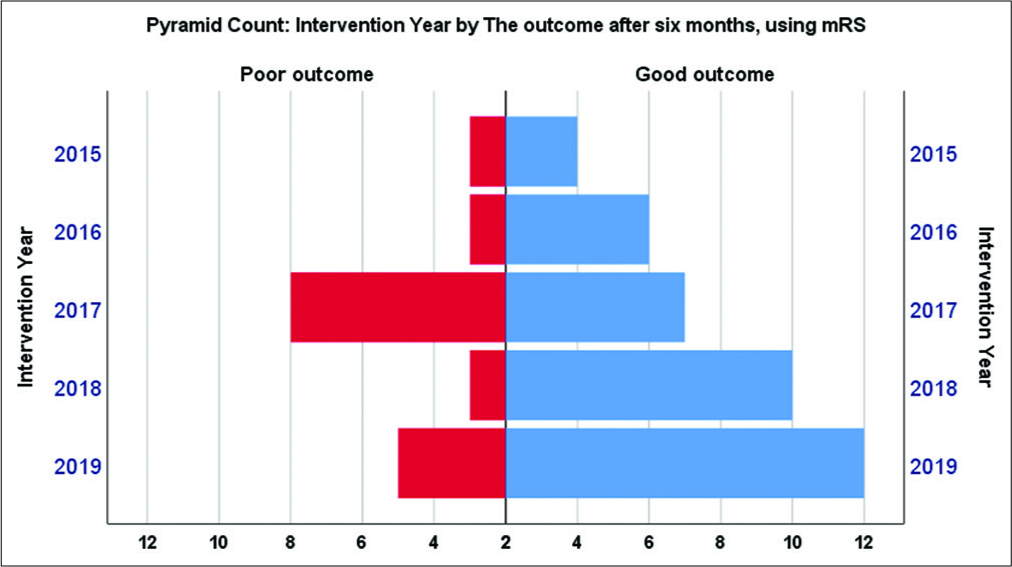
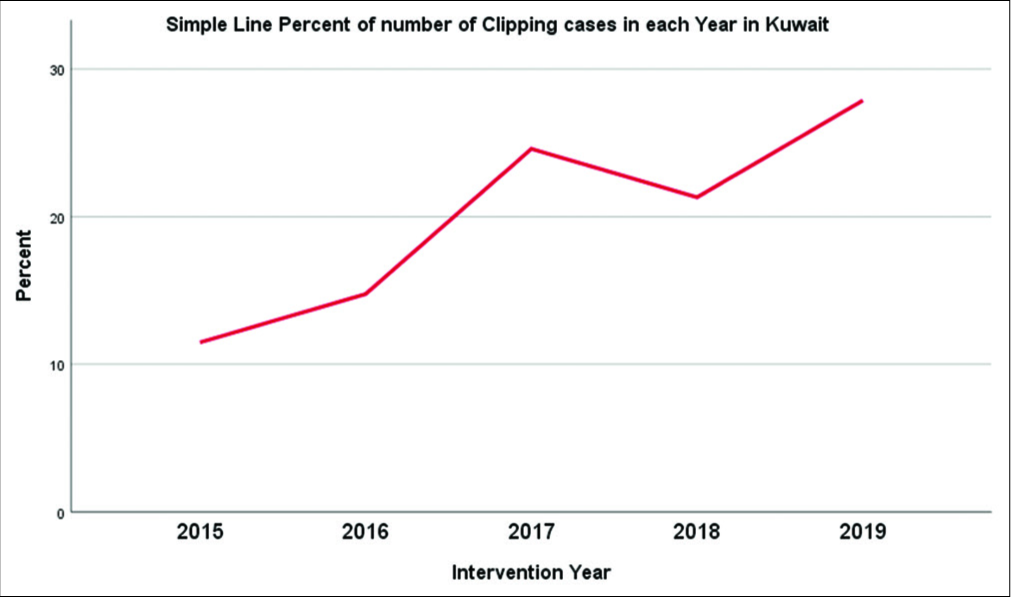
In 2015–2017, it appeared that outcomes did not reach a satisfactory level. After establishing the neurovascular unit in 2018, it seemed that the functional outcome has been significantly improved [
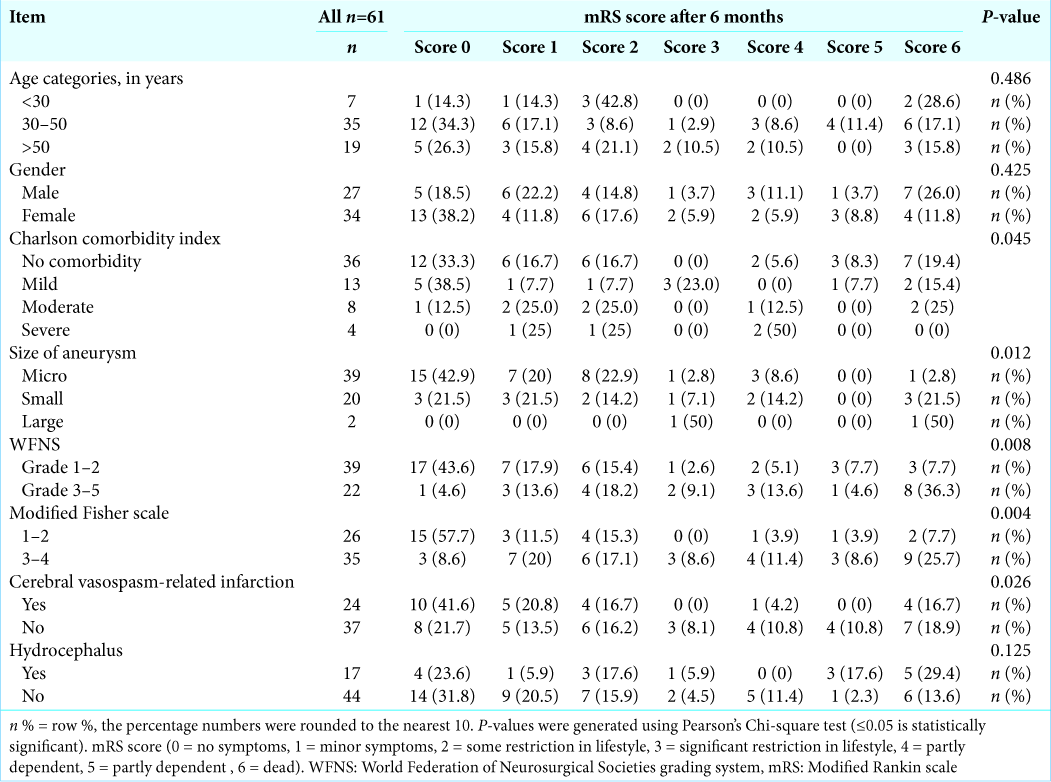
The association between the outcomes was tested to the categorical variables [
Participants who develop hydrocephalus had a poorer outcome, but this relation was not statistically remarked. The radiological vasospasm was noticed among 24 patients (39.3%). Unsurprisingly, the development of cerebral vasospasm was associated with a poor outcome.
DISCUSSION
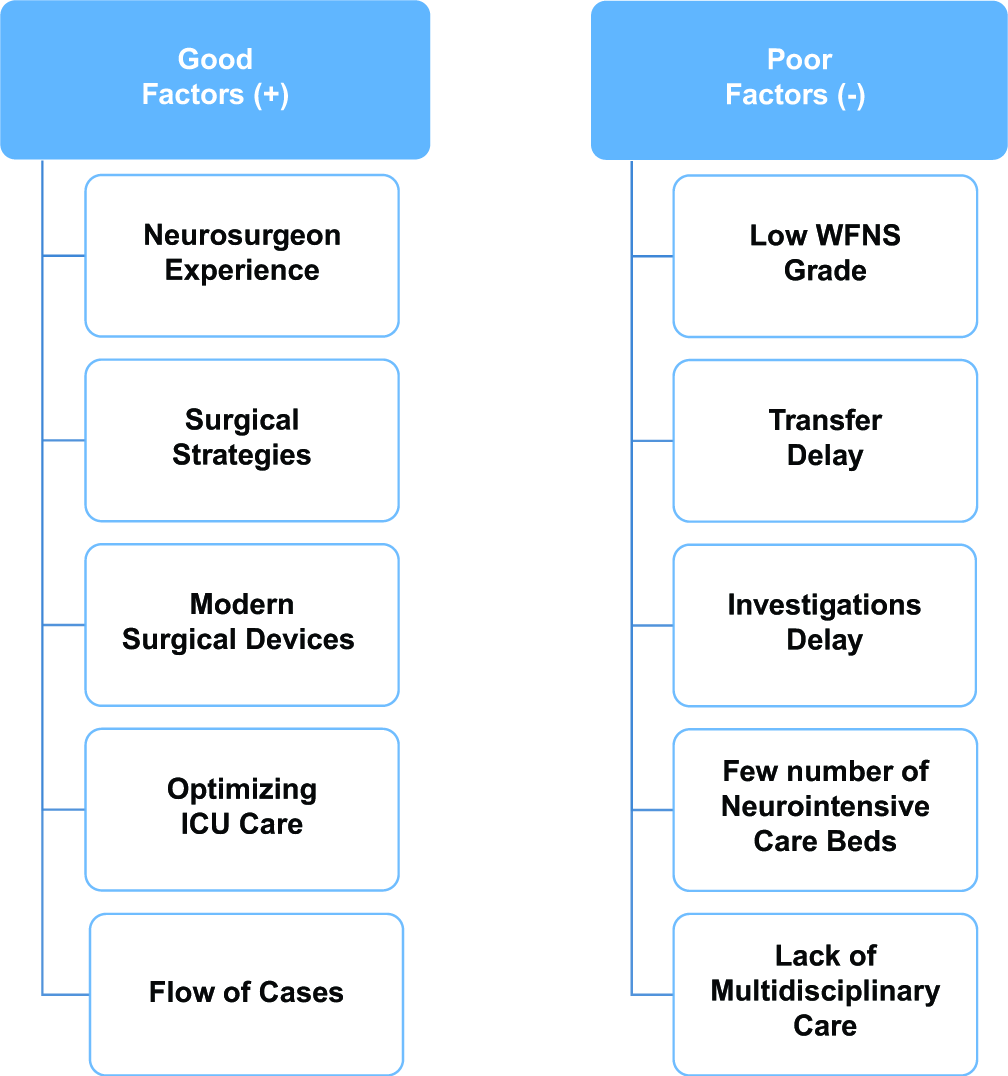
Establishing the cerebrovascular unit in 2018 has enhanced the functional outcome and decreased mortality. This could be explained by expanding the vascular experience in our department along with promoting surgical and postsurgical care.
Apparently, the surgical clipping rate and its success for aneurysmal SAH are different from one center to another worldwide. The different results depend on several variables. As expected, surgical experience, which is often measured by annual case volume, plays a major role.[
Clipping of cerebral aneurysm is one of the most complex surgical interventions. We believe that the experience of the surgeon is one of the most important factors to secure the aneurysm effectively. A large retrospective cohort performed in the USA explored that 37.6% of patients with aneurysmal SAH underwent clipping, and 62.4% were coiled.[
The literature has accepted that the management of aneurysmal SAH demands multiple efforts from different specialties.[
Intraoperative angiography helps place the clip and confirms parent vessel patency after securing the aneurysm, and it permits immediate clip revision when necessary.[
From the cohort, the annual mortality percentage after clipping was as follows: 28.5% in 2015, 11.1% in 2016, 40% in 2017, 7.7% in 2018, and 5.9% in 2019 [
We summarized a scheme of our experience in [
Preoperative neurological status to consider surgical intervention
In our center, we considered clipping for all SAH cases with WFNS grades from 1 to 5, except those with a GCS of less than 5. After stabilizing the cases with a very poor prognosis (GCS 3 or 4), the decision could be determined after the proper neurological status reassessment and angiography findings.
The cases in our sample were not amenable for coiling. All patients underwent cerebral angiography before the surgical intervention, except one case. We faced a condition of anterior communicating artery aneurysm that we found that it is rationale to operate it emergently based on the CT angiography only due to severe mass effect of the intracerebral hematoma, which caused a significant midline shift (mRS after the follow-up = 1).
Strengths and limitations
This is the first study in Kuwait and the Middle East region that questions this hypothesis. Moreover, we collected data from a relatively long duration (5 years). The follow-up to determine the outcome was for at least 6 months and was sufficient to decide the modified ranking score. The accuracy of data collection was completed in duplicate. In addition, a strict inclusion and exclusion criteria were applied for participant selection.
A major limitation is the retrospective design, creating a low possibility for imprecise data collection. However, the data were double-checked and revised to decrease the possibility of bias. This is an observational analysis, and there is still a chance of residual confounding. Another limitation is the relatively small sample size in relation to the long study duration.
CONCLUSION
There has been a considerable advance in microsurgical clipping experience in Kuwait. The recruitment of expertise, advancement of surgical techniques and intensive care, and the flow of cases have further improved our outcomes. We recommend considering the factors that we faced in our experience to optimize the management of SAH cases in neurosurgical centers. Larger multicenter studies to compare clipping and coiling outcomes are essential for better validation of both modalities in various centers.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank everyone who contributed to this project, especially for those who provided us with the data.
References
1. Abraham MK, Chang WW. Subarachnoid hemorrhage. Emerg Med Clin North Am. 2016. 34: 901-16
2. Ahmed SI, Javed G, Bareeqa SB, Samar SS, Shah A, Giani A. Endovascular coiling versus neurosurgical clipping for aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Cureus. 2019. 11: e4320
3. Bekelis K, Gottlieb D, Su Y, O’Malley AJ, Labropoulos N, Goodney P. Surgical clipping versus endovascular coiling for elderly patients presenting with subarachnoid hemorrhage. J Neurointerv Surg. 2016. 8: 913-8
4. Chang TR, Kowalski RG, Carhuapoma JR, Tamargo RJ, Naval NS. Impact of case volume on aneurysmal subarachnoid hemorrhage outcomes. J Crit Care. 2015. 30: 469-72
5. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994. 47: 1245-51
6. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2012. 43: 1711-37
7. Elewa MK. Endovascular coiling for cerebral aneurysm: Single-center experience in Egypt. Egypt J Neurol Psychiatr Neurosurg. 2018. 54: 33
8. Falk Delgado A, Andersson T, Delgado AF. Clinical outcome after surgical clipping or endovascular coiling for cerebral aneurysms: A pragmatic meta-analysis of randomized and non-randomized trials with short-and long-term follow-up. J Neurointerv Surg. 2017. 9: 264-77
9. Flett LM, Chandler CS, Giddings D, Gholkar A. Aneurysmal subarachnoid hemorrhage: Management strategies and clinical outcomes in a regional neuroscience center. AJNR Am J Neuroradiol. 2005. 26: 367-72
10. Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery. 2006. 59: 21-7
11. Hsu CE, Lin TK, Lee MH, Lee ST, Chang CN, Lin CL. The impact of surgical experience on major intraoperative aneurysm rupture and their consequences on outcome: A multivariate analysis of 538 microsurgical clipping cases. PLoS One. 2016. 11: e0151805
12. Lindgren A, Turner EB, Sillekens T, Meretoja A, Lee JM, Hemmen TM. Outcome after clipping and coiling for aneurysmal subarachnoid hemorrhage in clinical practice in Europe, USA, and Australia. Neurosurgery. 2019. 84: 1019-27
13. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005. 366: 809-17
14. Papadimitriou-Olivgeris M, Zotou A, Koutsileou K, Aretha D, Boulovana M, Vrettos T. Fatores de risco para mortalidade após hemorragia subaracnoidea: Estudo observacional retrospectivo [Risk factors for mortality after subarachnoid hemorrhage: A retrospective observational study]. Rev Bras Anestesiol. 2019. 69: 448-54
15. Rabinstein AA, Lanzino G, Wijdicks EF. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2010. 9: 504-19
16. .editors. Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988. 68: 985-6
17. Rumalla K, Lin M, Ding L, Gaddis M, Giannotta SL, Attenello FJ, Mack WJ. Risk factors for cerebral vasospasm in aneurysmal subarachnoid hemorrhage: A population-based study of 8346 patients. World Neurosurg. 2021. 145: e233-41
18. Seibert B, Tummala RP, Chow R, Faridar A, Mousavi SA, Divani AA. Intracranial aneurysms: Review of current treatment options and outcomes. Front Neurol. 2011. 2: 45
19. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P. Ten-year analysis of saccular aneurysms in the barrow ruptured aneurysm trial. J Neurosurg. 2019. 132: 771-6
20. Taheri Z, Harirchian MH, Ghanaati H, Khoshnevisan A, Salamati P, Miri M. Comparison of endovascular coiling and surgical clipping for the treatment of intracranial aneurysms: A prospective study. Iran J Neurol. 2015. 14: 22-8
21. Tang G, Cawley CM, Dion JE, Barrow DL. Intraoperative angiography during aneurysm surgery: A prospective evaluation of efficacy. J Neurosurg. 2002. 96: 993-9
22. Udy AA, Vladic C, Saxby ER, Cohen J, Delaney A, Flower O. Subarachnoid hemorrhage patients admitted to intensive care in Australia and New Zealand: A multicenter cohort analysis of in-hospital mortality over 15 years. Crit Care Med. 2017. 45: e138-45
23. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988. 19: 604-7
24. World Population Review, Kuwait Population. Available from: https://worldpopulationreview.com/countries/kuwait-population.