- Department of Neurosurgery, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho, Nagoya, Aichi, Japan.
DOI:10.25259/SNI_439_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Motoki Tanikawa, Hiroshi Yamada, Tomohiro Sakata, Mitsuhito Mase. Dosing interval adjustment of denosumab for the treatment of giant cell tumor of the sphenoid bone: A case report. 06-Nov-2020;11:370
How to cite this URL: Motoki Tanikawa, Hiroshi Yamada, Tomohiro Sakata, Mitsuhito Mase. Dosing interval adjustment of denosumab for the treatment of giant cell tumor of the sphenoid bone: A case report. 06-Nov-2020;11:370. Available from: https://surgicalneurologyint.com/surgicalint-articles/10379/
Abstract
Background: In the treatment of giant cell tumor of bone (GCTB), the efficacy and safety of denosumab, a receptor activator nuclear factor κ-B ligand inhibitor, has previously been demonstrated, especially for unresectable tumors. One of the current issues in denosumab treatment for unresectable GCTB is whether it can be discontinued, or whether the dosage or the dosing interval can safely be adjusted, if discontinuation is not possible, to avoid the occurrence of side effects.
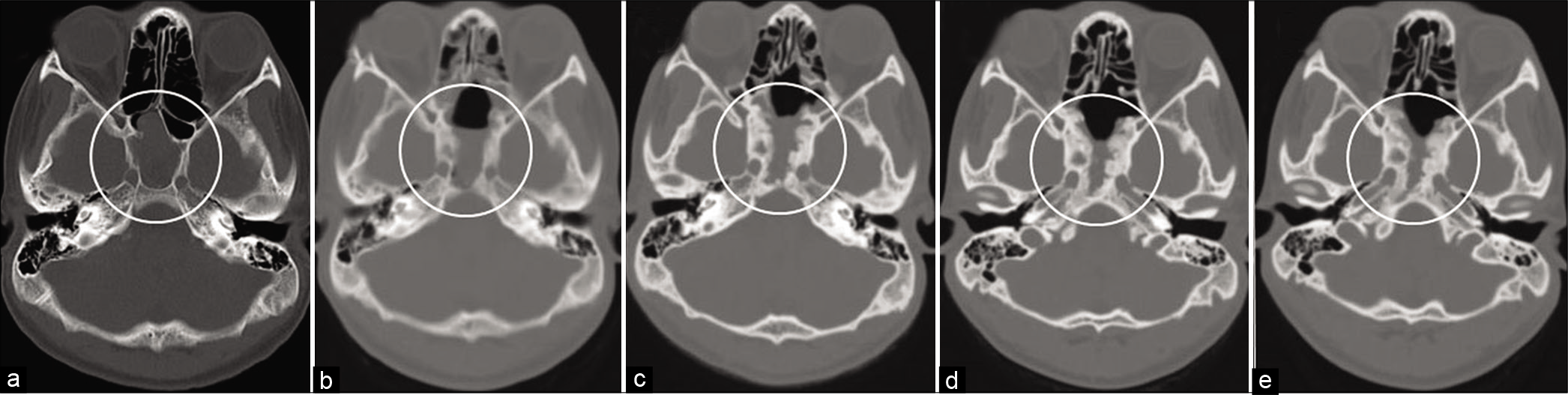
Case Description: A 15-year-old boy with diplopia was referred to our hospital after a space-occupying lesion in the sphenoid bone was found on head CT. Partial removal of the tumor was performed through an endoscopic endonasal approach, and pathological diagnosis was confirmed as GCTB. Thereafter, the patient received 120 mg subcutaneous injections of denosumab every 28 days for the first 2 years. Since bone formation was induced and sustained along with tumor reduction, the dosing interval was gradually extended, with 4 monthly dosing for the next 1 year, followed by 6 monthly dosing for the succeeding 2 years. With the extension of the dosing interval, the ossified tumor has regrown slightly, but within an acceptable range.
Conclusion: Discontinuation of denosumab treatment for unresectable GCTB was not thought to be possible for the current case due to the nature of the drug, as reported in the literature. Extending the dosing interval up to 6 monthly, as could be done safely in the current case, can be considered a useful and appropriate measure.
Keywords: Denosumab, Dosing interval, Giant cell tumor of bone, Sphenoid bone
INTRODUCTION
Giant cell tumor of bone (GCTB) is an uncommon, osteolytic tumor that occurs mainly in young adults (peak incidence ages, 20–40 years) with a slight female predominance (3:2).[
Although a complete surgical removal with invaded bone is generally the gold standard of treatment for GCTB,[
Clinical presentation
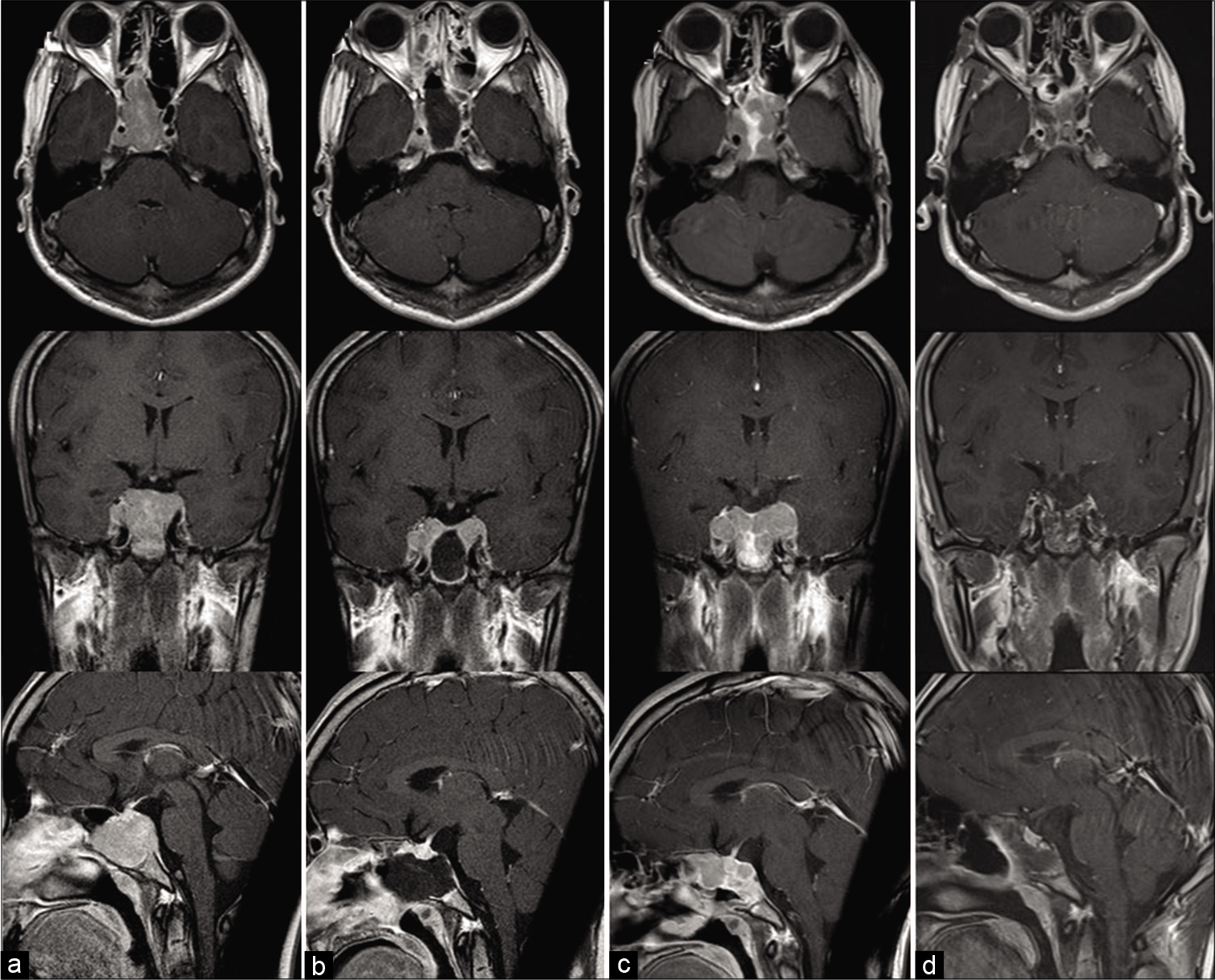
Informed consent was obtained from the patient and his guardian. A 15-year-old boy presented with diplopia which had an insidious onset, progressing over a period of 2 months. He was referred to our hospital after a space-occupying lesion was pointed out in the sphenoid bone on computed tomography scan (CT) of the head by an area doctor. On admission, neurological examination revealed bilateral abducens nerve palsy. CT and magnetic resonance imaging (MRI) showed a well-enhanced mass lesion in the sphenoid sinus, with osteolytic invasion of ethmoid cells anteriorly, the sellar floor, dorsum sellae, and posterior clinoid processes posteriorly, and the clivus inferiorly [
Figure 2:
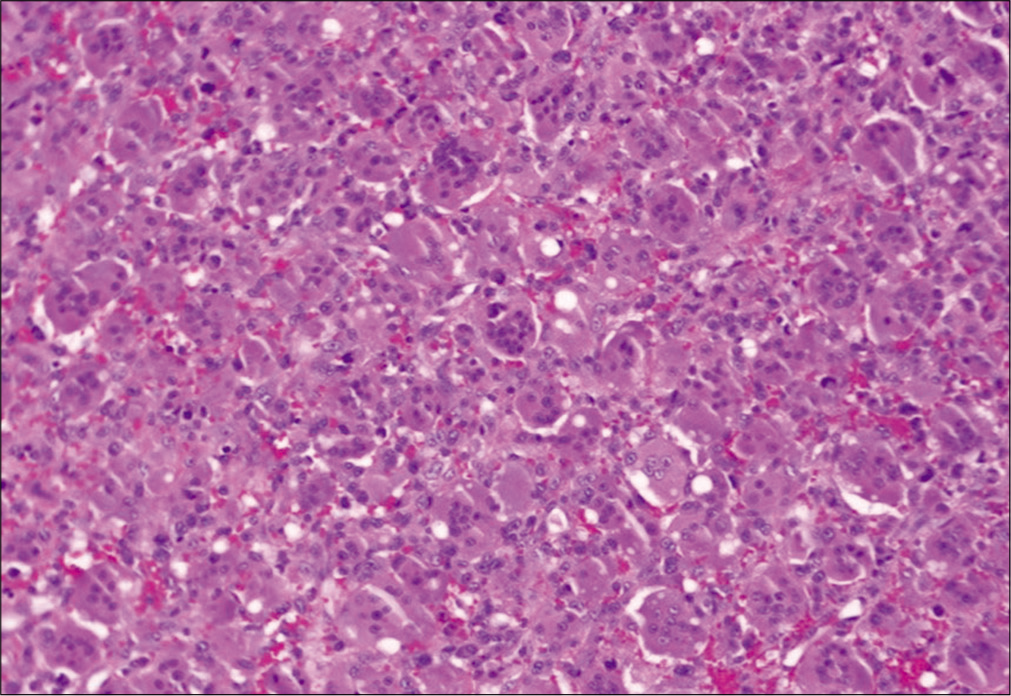
Photomicrograph of hematoxylin and eosin (H and E)-stained histopathologic specimens from a patient with a giant cell tumor of sphenoid bone, demonstrating numerous multinucleated osteoclast-like giant cells distributed diffusely among a background of neoplastic mononuclear stromal cells and mononuclear macrophage linage cells.
Denosumab treatment consisted of 120 mg subcutaneous injections every 28 days for the first 2 years, with additional doses on days 8 and 15 [
DISCUSSION
Although complete removal with invaded bone is the preferred treatment for GCTB,[
Radiotherapy is not promising for the management of GCTB. Although there are some reports describing tolerable controllability of radiotherapy in managing GCTB,[
GCTB has exhibited resistance to most conventional chemotherapeutic agents, including interferons, ifosfamide, doxorubicin, cyclophosphamide, cisplatin, and other like treatments.[
Denosumab has been establishing a firm position in the management of unresectable GCTB.[
CONCLUSION
Denosumab treatment was very useful as an adjuvant for unresectable GCTB in the skull base, as reported in the literature. It was considered that discontinuation of denosumab treatment for unresectable GCTB would be impossible due to the nature of the drug and that an adjustment of dosage or dosing interval according to each case after achieving the stabilization of the disease is both possible and important with long-term continuation of the drug, to avoid the occurrence of adverse effects. Extending the dosing interval up to 6 monthly, as could be done safely in the current case, can be considered a useful and appropriate measure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bardakhchyan S, Kager L, Danielyan S, Avagyan A, Karamyan N, Vardevanyan H. Denosumab treatment for progressive skull base giant cell tumor of bone in a 14 year old female-a case report and literature review. Ital J Pediatr. 2017. 43: 32
2. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised freedom trial and open-label extension. Lancet Diabetes Endocrinol. 2017. 5: 513-23
3. Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013. 14: 901-8
4. Dahlin DC. Caldwell lecture. Giant cell tumor of bone: Highlights of 407 cases. AJR Am J Roentgenol. 1985. 144: 955-60
5. Feigenberg SJ, Marcus RB, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Radiation therapy for giant cell tumors of bone. Clin Orthop Relat Res. 2003. 411: 207-16
6. Gamboa NT, Ronna B, Gamboa CT, Palmer CA, Park MS, Gurgel RK. Giant cell tumor of the lateral skull base: Diagnostic and management options. J Neurol Surg Rep. 2018. 79: e41-54
7. Goto Y, Furuno Y, Kawabe T, Ohwa K, Tatsuzawa K, Sasajima H. Treatment of a skull-base giant cell tumor with endoscopic endonasal resection and denosumab: Case report. J Neurosurg. 2017. 126: 431-4
8. Gouin F, Rochwerger AR, di Marco A, Rosset P, Bonnevialle P, Fiorenza F. Adjuvant treatment with zoledronic acid after extensive curettage for giant cell tumours of bone. Eur J Cancer. 2014. 50: 2425-31
9. Gupta R, Seethalakshmi V, Jambhekar NA, Prabhudesai S, Merchant N, Puri A. Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in Western India. Ann Diagn Pathol. 2008. 12: 239-48
10. Lau CP, Huang L, Wong KC, Kumta SM. Comparison of the anti-tumor effects of denosumab and zoledronic acid on the neoplastic stromal cells of giant cell tumor of bone. Connect Tissue Res. 2013. 54: 439-49
11. Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006. 29: 96-9
12. Nishimura S, Hashimoto K, Tan A, Yagyu Y, Akagi M. Successful treatment with denosumab in a patient with sacral giant cell tumor of bone refractory to combination therapy with arterial embolization and zoledronic acid: A case report. Mol Clin Oncol. 2017. 6: 307-10
13. Thomas DM, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010. 11: 275-80
14. Thomas DM, Skubitz KM. Giant cell tumour of bone. Curr Opin Oncol. 2009. 21: 338-44
15. van der Heijden L, Dijkstra PD, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer. 2017. 77: 75-83
16. van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS. The clinical approach toward giant cell tumor of bone. Oncologist. 2014. 19: 550-61