- Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- Department of Radiation Oncology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Bishan Dass Radotra, Department of Histopathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_307_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mayur Parkhi1, Debajyoti Chatterjee1, Bishan Dass Radotra1, Amanjit Bal1, Budhi Singh Yadav2, Manjul Tripathi3. Double-hit and double-expressor primary central nervous system lymphoma: Experience from North India of an infrequent but aggressive variant. 12-May-2023;14:172
How to cite this URL: Mayur Parkhi1, Debajyoti Chatterjee1, Bishan Dass Radotra1, Amanjit Bal1, Budhi Singh Yadav2, Manjul Tripathi3. Double-hit and double-expressor primary central nervous system lymphoma: Experience from North India of an infrequent but aggressive variant. 12-May-2023;14:172. Available from: https://surgicalneurologyint.com/surgicalint-articles/12318/
Abstract
Background: High-grade non-Hodgkin B-cell lymphoma is an aggressive mature B-cell lymphoma that depicts poor treatment response and worse prognosis. The presence of MYC and B-cell lymphoma 2 (BCL2) and/or B-cell lymphoma 6 (BCL6) rearrangements qualifies for triple-hit and double-hit lymphomas (THL/DHL), respectively. We attempted to explore the incidence, distribution, and clinical characteristics of the primary high-grade B-cell lymphoma of the central nervous system (CNS) in our cohort from North India.
Methods: All the histologically confirmed cases of primary CNS diffuse large B-cell lymphoma (PCNS-DLBCL) over a period of 8 years were included. Cases showing MYC and BCL2 and/or BCL6 expression on immunohistochemistry (IHC) (double- or triple-expressor) were further analyzed by fluorescence in situ hybridization for MYC, BCL2 and /or BCL6 rearrangements. The results were correlated with other clinical and pathological parameters, and outcome.
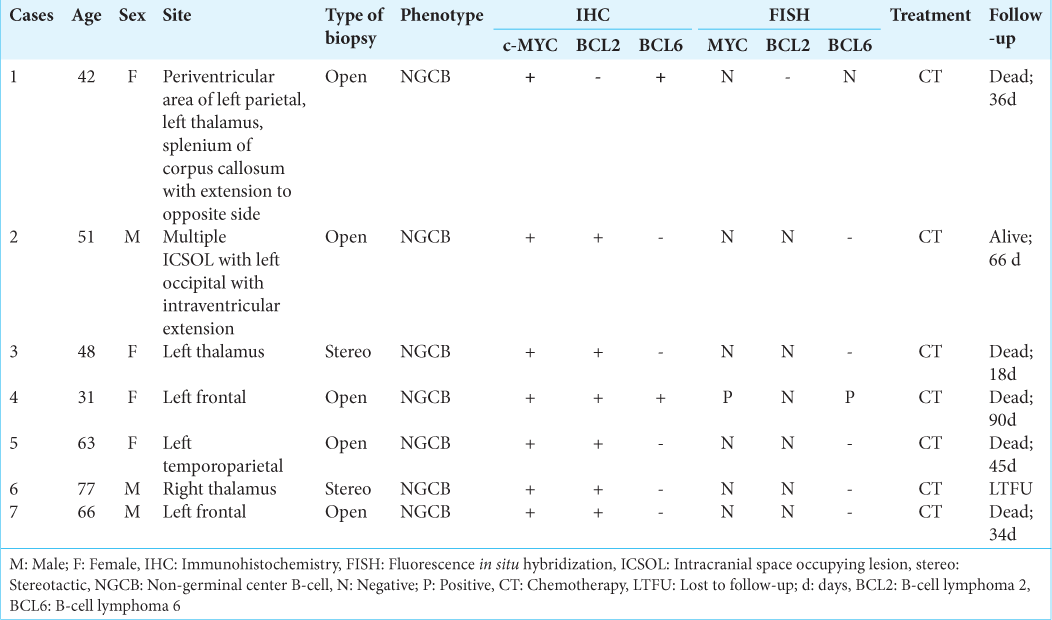
Results: Of total 117 cases of PCNS-DLBCL, there were seven (5.9%) cases of double/triple-expressor lymphomas (DEL/TEL) (six double- and one triple-expressor) with median age of 51 years (age range: 31–77 years) and slight female predilection. All were located supratentorially and were of non-geminal center B-cell phenotype. Only triple-expressor case (MYC+/BCL2+/BCL6+) demonstrated concurrent rearrangements for MYC and BCL6 genes indicating DHL (n = 1, 0.85%), while none of the double-expressors (n = 6) showed MYC, BCL2, or BCL6 rearrangements. The mean overall survival of the DEL/TEL was 48.2 days.
Conclusion: DEL/TEL and DHL are uncommon in CNS; mostly located supratentorially and are associated with poor outcome. MYC, BCL2, and BCL6 IHC can be used as an effective screening strategy for ruling out double/ triple-expressor PCNS-DLBCLs.
Keywords: Central nervous system, Double-hit lymphoma, Fluorescence in situ hybridization, High-grade B-cell non-Hodgkin lymphoma, Immunohistochemistry
INTRODUCTION
Primary central nervous system (CNS) lymphomas are extranodal, malignant non-Hodgkin lymphomas (NHL) that are principally limited to the brain, leptomeninges, eyes or spinal cord, without systemic involvement.[
MATERIALS AND METHODS
All histologically diagnosed cases of primary CNS (PCNSDLBCL) over a period of 8 years (July 2013–June 2021) in our department were evaluated. Approval from the Institute Ethic Committee was obtained (reference no. NK/8003/ Study/864). Majority of the cases were already worked-up for Han’s algorithm as a part of two earlier published studies.[
Immunohistochemistry (IHC)
For diagnosis, subtyping and double/triple expressor status, the following antibodies were used: Glial fibrillary acidic protein (clone EP672Y, Cell Marque, dilution 1:100), leukocyte common antigen (clone 2B11 + PD7/26, Dako, dilution 1:100), CD3 (Rabbit polyclonal, Cell Marque, dilution 1:500), CD20 (clone L26, Dako, dilution 1:300), CD10 (clone 56C6, Cell Marque, dilution 1:20), B-cell lymphoma 6 (BCL6; cone G1191E/A8, Cell Marque, dilution 1:300), Multiple Myeloma 1 (clone MRQ-43, Cell Marque, dilution 1:300), B-cell lymphoma 2 (BCL2; clone 124, Dako, dilution 1:50), MYC (clone EP121, Cell Marque, dilution 1:50), and Ki-67 (clone SP6, Cell Marque, dilution 1:300). These were performed on Ventana, Biotek automated system with appropriate positive and negative controls run concurrently. Like the previous study, the standard universal cutoffs for positivity were considered.[
The IHC slides were independently evaluated by two pathologists, who were blinded to the clinical data. The cutoff for MYC, BCL6, and BCL2 positivity were taken as 40%, 50%, and 50%, respectively. Cases showing MYC and BCL2 and/or BCL6 expression (double- or triple-expressor lymphoma, DEL/TEL) were further analyzed by fluorescence in situ hybridization (FISH) for MYC, BCL2, and BCL6 rearrangements.
FISH
FISH was performed on formalin-fixed, paraffin-embedded tissue for MYC, BCL2, and BCL6 rearrangements using Dual Color Break Apart Probe (Abbott/Vysis). The signals were analyzed on 3–4 μm sections of all respective blocks by fluorescent microscope (Olympus BX53) using appropriate filters and images were assessed by Genesis software (version 8.1.0.47741). Overlapped nuclei, ill-defined borders, or degenerated nuclei were excluded from evaluation. Minimum 100 interphase nuclei were scored. A positivity for rearrangement was defined as >15% of tumor cells with a split or single red signal.
RESULTS
A total of 117 histopathologically confirmed PCNS-DLBCL cases were included in this study. The median age of presentation was 55 years (age range: 19–85 years) with male-to-female ratio of 1.65:1. Ninety-five cases were located in the supratentorial compartment (81.19%). Using Hans’ algorithm, the cases were divided into non-GCB (n = 96; 82.05%), GCB (n = 14; 11.96%), and unclassified (n = 7; 5.98%) categories.
MYC, BCL2, and BCL6 expression by IHC
There were six double-expressor and one TEL. Among the double-expressor cases (n = 6), the overexpression for both MYC and BCL2 protein (MYC+/BCL2+) was seen in five cases [
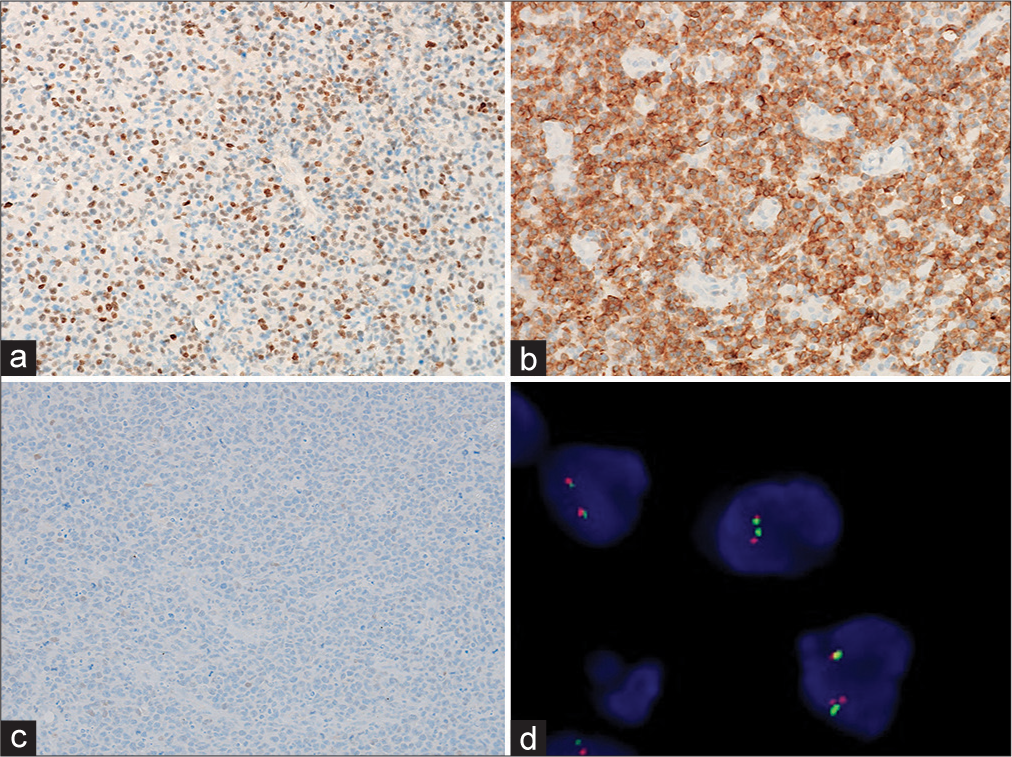
Figure 1:
Primary double-expressor central nervous system lymphoma (Case 1). (a) Strong nuclear positivity for MYC (>40%; peroxidase; ×200). (b) B-cell lymphoma 2 (BCL2) showing diffuse cytoplasmic expression (>50%; peroxidase; ×200). (c) B-cell lymphoma 6 (BCL6) showing no positivity (peroxidase; ×200). (d) No MYC rearrangement on fluorescence in situ hybridization. Furthermore, no rearrangements for BCL2 and BCL6 genes (images not provided).
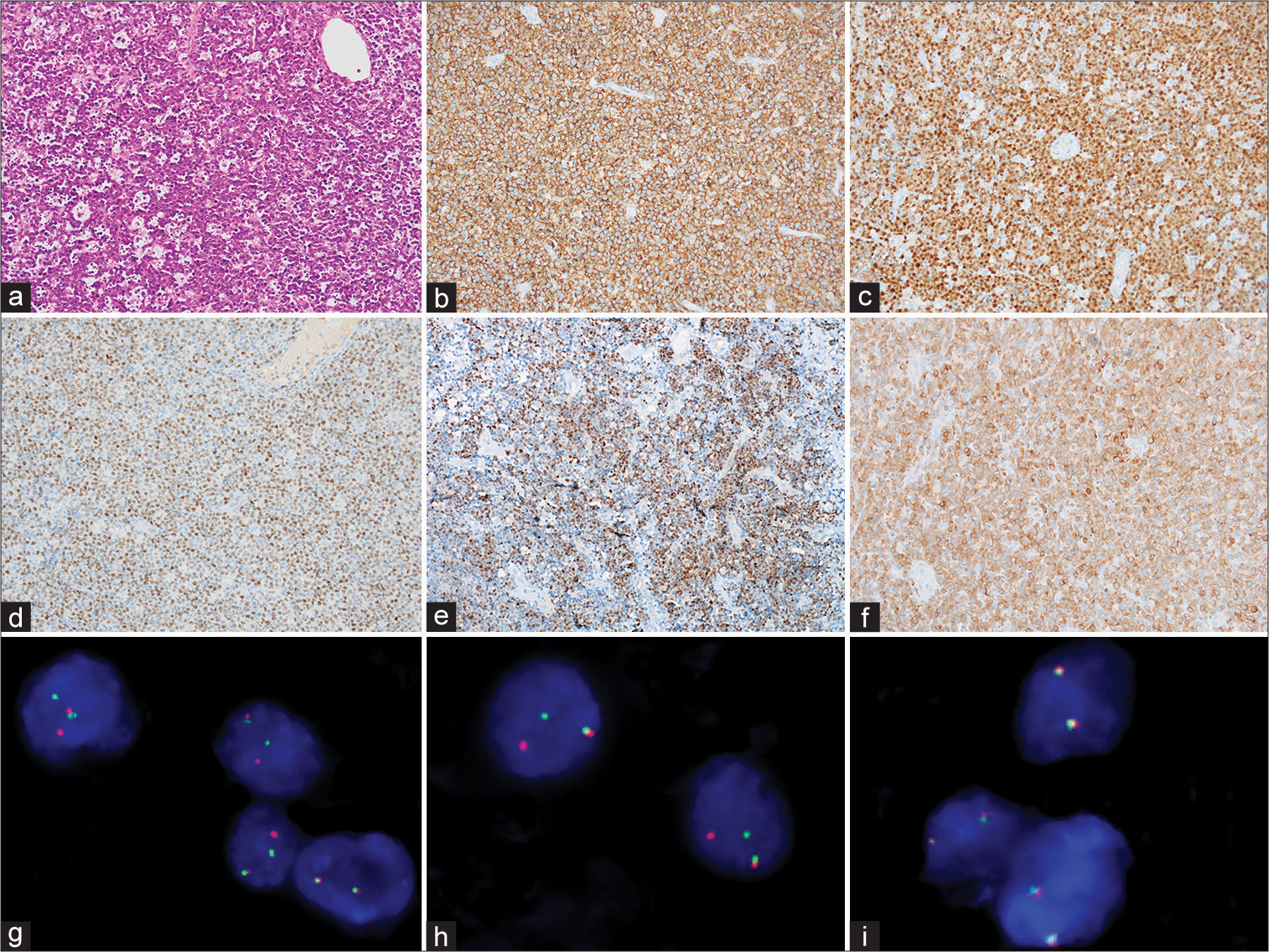
Figure 2:
Primary double-hit central nervous system lymphoma (Case 4). (a) High-grade B-cell lymphoma with starry-sky growth pattern and centroblastic morphology (hematoxyline and eosin; ×200). (b) CD20 showing diffuse membranous positivity (peroxidase; ×200). (c) Diffuse nuclear positivity for multiple myeloma 1 (>30%; peroxidase; ×200). (d) Diffuse nuclear positivity for MYC (>40%; peroxidase; ×200). (e) Variable nuclear positivity for B-cell lymphoma 6 (BCL6) (>50%; peroxidase; ×200). (f) Diffuse cytoplasmic positivity for B-cell lymphoma 2 (BCL2) (>50%; peroxidase; ×200). (g-i) Fluorescence in situ hybridization using dual color break apart probe shows concurrent rearrangements for MYC (g) and BCL6 (h) genes but no BCL2 gene rearrangement (i).
MYC, BCL2, and BCL6 rearrangements by FISH
FISH for rearrangement was performed on these seven cases of DEL/TEL. The triple-expressor case (MYC+/BCL2+/BCL6+) demonstrated concurrent rearrangement for MYC and BCL6 indicating DHL [
Treatment and follow-up
One case of DEL was lost to follow-up. Remaining six patients received standard chemotherapy (high-dose methotrexate [poly-/monochemotherapy] + rituximab). Five patients (including the DHL) died, and one was alive at last follow-up [
DISCUSSION
DHL is a subtype of high-grade B-cell NHL that shows MYC rearrangement and additional rearrangement of known oncogenes. BCL2 acts as the most often co-rearranged gene (75%), followed by CCND1 (13%), BCL6 (10%), and BCL3 (2%).[
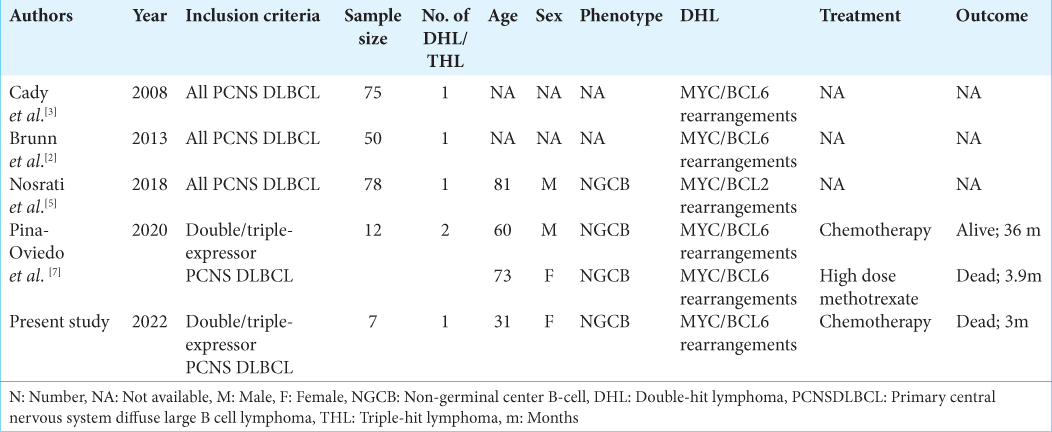
In this study, we came across a single case of DHL (MYC/ BCL6 concurrent rearrangement) in a young female, who presented with left frontal mass. The biopsy showed features of DLBCL with centroblastic morphology which was of non-GCB phenotype. She received chemotherapy regime (high dose methotrexate) but succumbed to her illness after 90 days of follow-up. Only five cases of DHL have been documented in the CNS till date, while there is no report of THL [
CONCLUSION
Primary DEL and DHL are extremely rare in the CNS. Compared to the systemic DHL where MYC/BCL2 rearrangement is seen in the majority, CNS DHL cases mostly show MYC/BCL6 rearrangement. In this study, DEL and DHL in CNS uniformly showed poor outcome. IHC for MYC, BCL2, and BCL6 is an effective screening strategy to exclude the DEL and DHL cases in PCNS-DLBCL, since such cases may require more aggressive therapy. Thus, we recommend performing this panel of IHC in all cases of PCNS-DLBCL, even if FISH is not available.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Aukema SM, Siebert R, Schuuring E, van Imhoff GW, KluinNelemans HC, Boerma EJ. Double-hit B-cell lymphomas. Blood. 2011. 117: 2319-31
2. Brunn A, Nagel I, Montesinos-Rongen M, Klapper W, Vater I, Paulus W. Frequent triple-hit expression of MYC, BCL2, and BCL6 in primary lymphoma of the central nervous system and absence of a favorable MYC(low)BCL2 (low) subgroup may underlie the inferior prognosis as compared to systemic diffuse large B cell lymphomas. Acta Neuropathol. 2013. 126: 603-5
3. Cady FM, O’Neill BP, Law ME, Decker PA, Kurtz DM, Giannini C. Del(6)(q22) and BCL6 rearrangements in primary CNS lymphoma are indicators of an aggressive clinical course. J Clin Oncol. 2008. 26: 4814-9
4. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004. 103: 275-82
5. Nosrati A, Monabati A, Sadeghipour A, Radmanesh F, Safaei A, Movahedinia S. MYC, BCL2, and BCL6 rearrangements in primary central nervous system lymphoma of large B cell type. Ann Hematol. 2019. 98: 169-73
6. Ok CY, Medeiros LJ. High-grade B-cell lymphoma: A term re-purposed in the revised WHO classification. Pathology. 2020. 52: 68-77
7. Pina-Oviedo S, Bellamy WT, Gokden M. Analysis of primary central nervous system large B-cell lymphoma in the era of high-grade B-cell lymphoma: Detection of two cases with MYC and BCL6 rearrangements in a cohort of 12 cases. Ann Diagn Pathol. 2020. 48: 151610
8. Parkhi M, Chatterjee D, Bal A, Vias P, Yadav BS, Prakash G. Prognostic implications of the tumor immune microenvironment and immune checkpoint pathway in primary central nervous system diffuse large B-cell lymphoma in the North Indian population. APMIS. 2022. 130: 82-94
9. Radotra BD, Parkhi M, Chatterjee D, Yadav BS, Ballari NR, Prakash G. Clinicopathological features of primary central nervous system diffuse large B cell lymphoma: Experience from a Tertiary Center in North India. Surg Neurol Int. 2020. 11: 424
10. Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017. 31: 37-42
11. Riedell PA, Smith SM. Double hit and double expressors in lymphoma: Definition and treatment. Cancer. 2018. 124: 4622-32
12. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. Revised. Lyon, France: IARC Press; 2017. p.
13. Thirunavukkarasu B, Bal A, Prakash G, Malhotra P, Singh H, Das A. Screening strategy for detecting double-hit lymphoma in a resource-limited setting. Appl Immunohistochem Mol Morphol. 2022. 30: 49-55
14. Villa D, Tan KL, Steidl C, Ben-Neriah S, Al Moosawi M, Shenkier TN. Molecular features of a large cohort of primary central nervous system lymphoma using tissue microarray. Blood Adv. 2019. 3: 3953-61
15. Yuan XG, Huang YR, Yu T, Xu Y, Liang Y, Zhang XH. Primary central nervous system lymphoma in China: A single-center retrospective analysis of 167 cases. Ann Hematol. 2020. 99: 93-104