- Department of Neuroradiology, ISNB - IRCCS, Bologna, Emilia-Romagna, Italy.
- Department of Pediatrics, Maggiore Hospital, ISNB - IRCCS, Bologna, Emilia-Romagna, Italy.
- Departments of Anaesthesiology ISNB - IRCCS, Bologna, Emilia-Romagna, Italy.
- Departments of Neurosurgery, ISNB - IRCCS, Bologna, Emilia-Romagna, Italy.
Correspondence Address:
Martino Cellerini
Department of Pediatrics, Maggiore Hospital, ISNB - IRCCS, Bologna, Emilia-Romagna, Italy.
DOI:10.25259/SNI_236_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Martino Cellerini1, Rosa Francavilla2, Caterina Testoni3, Monica Maffei1, Mino Zucchelli4, Chiara Ghizzi2. Dural sinus mechanical thrombectomy and continuous local rt-PA infusion in a child with refractory intracranial hypertension and progressive visual loss: A case report. 21-Aug-2020;11:253
How to cite this URL: Martino Cellerini1, Rosa Francavilla2, Caterina Testoni3, Monica Maffei1, Mino Zucchelli4, Chiara Ghizzi2. Dural sinus mechanical thrombectomy and continuous local rt-PA infusion in a child with refractory intracranial hypertension and progressive visual loss: A case report. 21-Aug-2020;11:253. Available from: https://surgicalneurologyint.com/surgicalint-articles/10222/
Abstract
Background: Children with intracranial hypertension are at risk for visual loss and their visual function must be closely monitored. Surgery with the insertion of a ventriculoperitoneal shunt is imperative when vision is threatened.
Case Description: Herein, we report a case of a 5-year-old boy whose refractory intracranial hypertension and severe, progressive visual loss (secondary to a chronic, otogenic, right sigmoid sinus thrombosis, and a contralateral sinus tight stenosis) were resolved by a combination of continuous (6 h), locoregional, infusion of recombinant tissue plasminogen activator (rt-PA), and mechanical thrombectomy.
Conclusion: The association of in loco and continuous infusion of recombinant tissue plasminogen activator (rt- PA) with mechanical thrombectomy resulted in effective in partially reopening the occluded sinus and facilitating a good clinical recovery. This combined endovascular approach may represent an alternative, less invasive, therapeutic option to surgery in children with intracranial hypertension caused by chronic cerebral venous sinus thrombosis.
Keywords: Dural sinus thrombosis, Intracranial hypertension, Recombinant tissue plasminogen activator, Thrombectomy, Vision
INTRODUCTION
This paper would like to draw attention to a possible alternative therapeutic option to surgery in children with refractory intracranial hypertension secondary to cerebral venous sinus thrombosis (CVST) and progressive visual deterioration.
CASE DESCRIPTION
A 5-year-old boy with a more than 2 months history of otogenic CVST of the right sigmoid sinus was referred by pediatrician consultants. Despite adequate antibiotics and anticoagulant therapy, the child developed progressive bilateral papilledema and visual loss. An MR imaging (MRI) – MR venography (MRV) of the head showed a thrombotic occlusion of the right transverse-sigmoid sinus associated with a tight stenosis of the left transverse sinus [
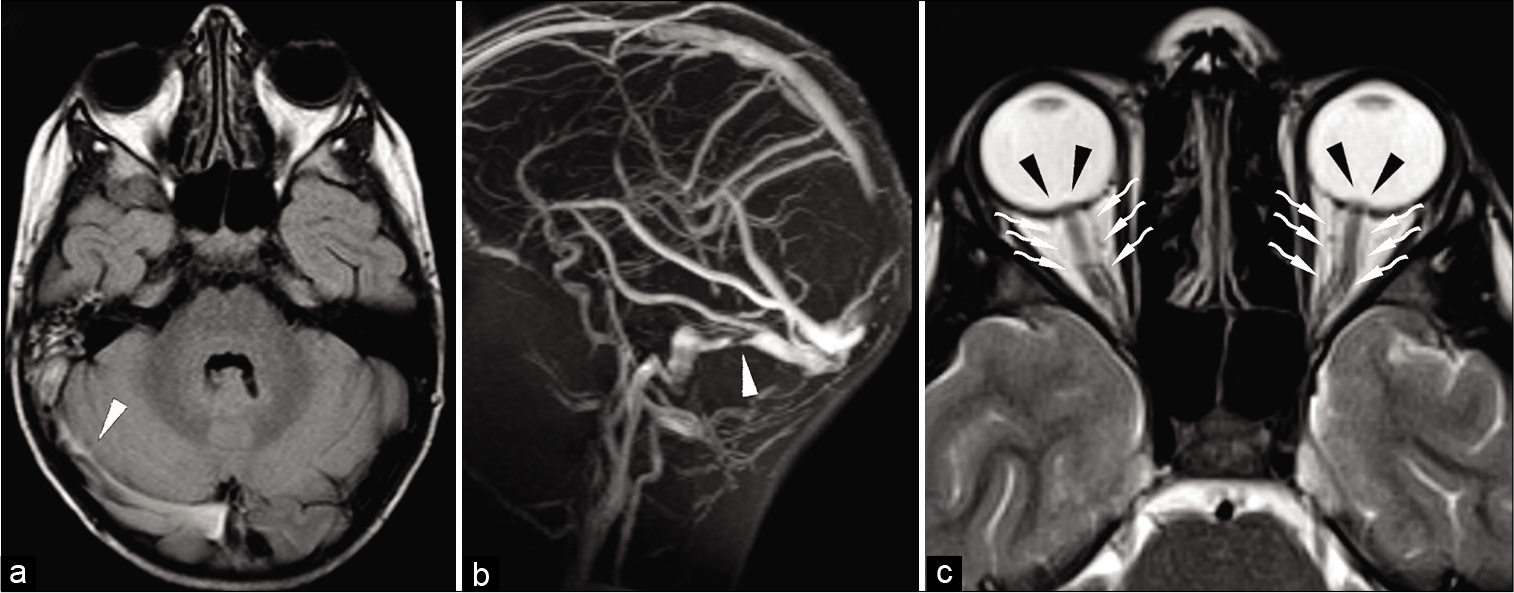
Figure 1:
MR imaging (a and c) and MR venogram in the sagittal view (b). An abnormal signal in the right transverse-sigmoid sinus (white arrowhead), associated with inflammatory changes in the right mastoid is noted in a. Absence of flow signal of a sigmoid sinus and a tight narrowing of the contralateral transverse sinus (white arrowhead) are depicted in b. Bilateral enlargement of the optic nerve sheaths (white waved arrowheads) and bulging of the optic discs (black arrowheads) are depicted in c. Appearances are consistent with an otogenic, right transverse-sigmoid sinus thrombotic occlusion with contralateral transverse sinus tight stenosis and indirect signs of an increased intracranial pressure.
An informed consent signed by both parents was obtained, and the procedure was performed under general anesthetics on a biplanar angiography system (Philips Allura). A 7F guider was placed in the right jugular bulb and a diagnostic 4F catheter in the right internal carotid artery (ICA) through a femoral (venous 7F and arterial 4F) approach. After several attempts, we managed to cross the occluded segment of the sinus with a 016” guide and a 021” microcatheter. A 6 × 30 mm Solitaire (Medtronic) stent- retriever was then delivered across the thrombosed sinus, unsheathed and, after a few minutes, wait, trashed out. A second pass with the stent-retriever was repeated: each time only small and scanty clot fragments were retrieved. While attempting to cross the occlusion for the third time, the child presented a transient tachyarrhythmia (200 bpm) and hypotension requiring the anesthesiologist’s assistance with a quick resolution of the event. Once the child was hemodynamically back to normal, a dynaCT was obtained, and it resulted in normal appearances. Subsequent check angiograms showed an unsatisfactory partial reopening of the occluded sinus [
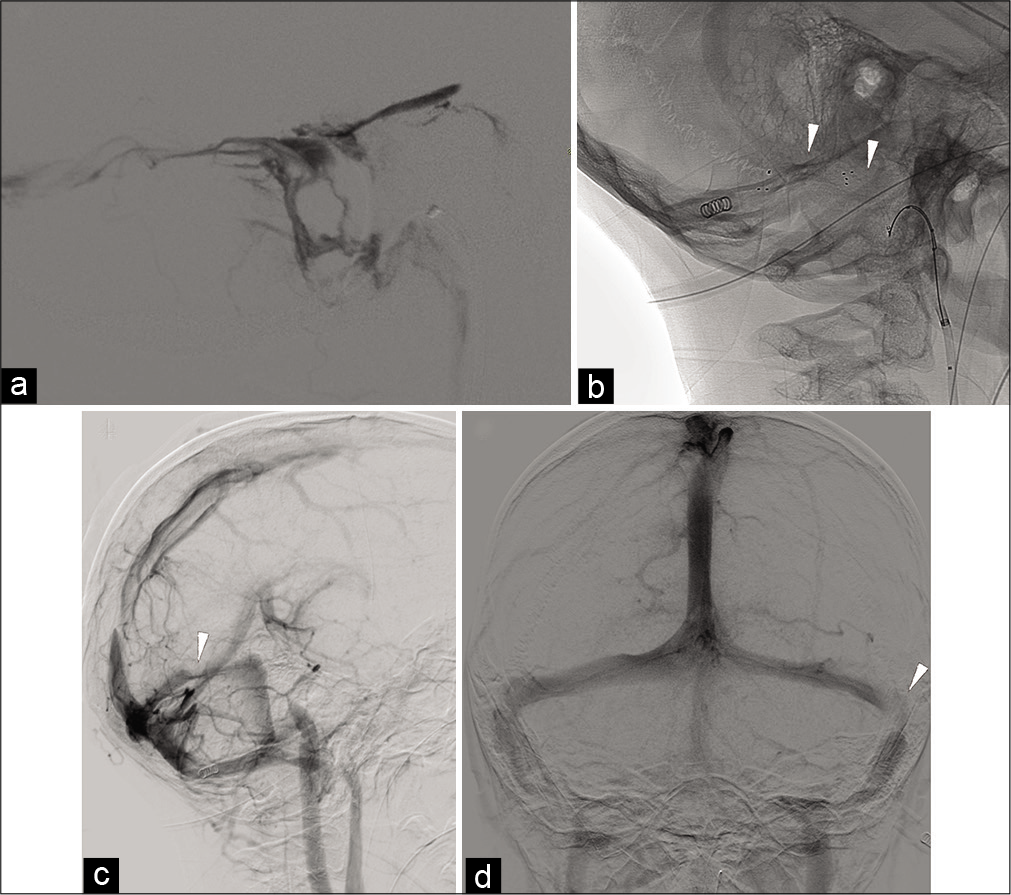
Figure 2:
DSA Internal jugular vein retrograde injection in the LL view (a), DSA unsubtracted mask in the lateral oblique view (b), DSA, right CCA injection, late venous phase in the lateral oblique (c), and AP views (d). Unsatisfactory, partial recanalization of the lateral transverse and sigmoid sinus at the end of the first endovascular procedure is depicted in a. The following day, mechanical thrombectomy with a double stent-retriever technique (b, white arrowheads) resulted in a satisfactory, although partial, reopening of the transverse and sigmoid sinuses (c and d). Note severe stenosis of the left lateral transverse sinus (white arrowheads in c and d).
DISCUSSION
The aforementioned case opens up new therapeutic options in children with refractory intracranial hypertension from CVST. In children, a systematic review of the literature identified nine children with acute CVST, treated using several endovascular methods, including local rt-PA, microguidewire and catheter disruption, balloon angioplasty, and thromboaspiration.[
Alternative therapeutic options were discussed, such as balloon angioplasty (PTA with/without stenting) of the contralateral sinus and CSF diversion.
The first option (PTA with/without stenting) was considered too invasive in a 5-year-old child taking into account that the sole PTA would have most likely resulted in a temporary improvement of the cerebral venous outflow. A disadvantage of venous sinus stenting would have been the necessity for temporary antiplatelet therapy in a young child, which limits his ability to participate in contact sports and predisposes him to a greater hemorrhage risk from trauma. Furthermore, deployment of a life-long foreign body in a 5-year-old child was not justified in the light of the incompletely understood long-term outcomes (what if after PTA and stenting the intracranial hypertension failed to improve?). In summary, the associated risk of the endovascular procedure and the antiplatelet therapy was considered to exceed the possible benefit.
As for the 2nd option, it is known that CSF diversion procedures are the main surgical treatment for patients with nonobstructive hydrocephalus because they are quite effective in improving and resolving clinical manifestations. However, surgical CSF diversion is associated with complications requiring revisions (e.g., CSF shunt malfunctioning and infections).[
In children, there are only scant reports of intrasinus rt-PA infusion, the rates, and total dose of the fibrinolytic differing greatly as well as the outcomes. In Waugh series of six patients, a continuous infusion of rt-PA was administered over a mean of 49 hours, with a maximum dose of 60 mg. In their series, 5/6 patients demonstrated hemorrhagic complications. Another series of three patients demonstrated a favorable outcome in 2/3 patients.[
Finally, in refractory intracranial hypertension, it is still an issue of hot debate whether the dural sinus narrowing is structural or secondary to the increased intracranial pressure. In our case, the stenosis of the contralateral transverse sinus was still present on the follow-up MRI 3 weeks later, when the child was discharged home with a substantial clinical recovery and resolution of the radiological signs of intracranial hypertension. Although no invasive measurement of the CSF pressure was obtained, follow-up MRI appearances were consistent with a significant decrease of the intracranial pressure, suggesting a possible structural cause of the left transverse sinus stenosis. Further, clinical and MRI follow-ups are planned to check for the recovery of the visual deficits and the possible development of idiopathic intracranial hypertension or dural AV fistulas.
CONCLUSION
Mechanical thrombectomy with a double stent-retrievers technique following a continuous, local, and 6 h infusion of rt-PA resolved intracranial hypertension and progressive visual loss in a 5-year-old child with a chronic otogenic transverse-sigmoid sinus occlusion. Mechanical thrombectomy with a double stent-retrievers with/without local infusion of rt-PA may represent a viable, less invasive and expensive, and alternative therapeutic option to surgery. However, due to the intra-periprocedural hemorrhagic risks, this technique should be reserved for only the most carefully selected cases of idiopathic intracranial hypertension secondary to chronic CVST.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bynke G, Zemack G, Bynke H, Romner B. Ventriculoperitoneal shunting for idiopathic intracranial hypertension. Neurology. 2004. 63: 1314-6
2. Gebara BM, Goetting MG, Wang AM. Dural sinus thrombosis complicating subclavian vein catheterization: Treatment with local thrombolysis. Pediatrics. 1995. 95: 138-40
3. Griesemer DA, Theodorou AA, Berg RA, Spera TD. Local fibrinolysis in cerebral venous thrombosis. Pediatr Neurol. 1994. 10: 78-80
4. Klisch J, Sychra V, Strasilla C, Taschner CA, Reinhard M, Urbach H. Double solitaire mechanical thrombectomy in acute stroke: Effective rescue strategy for refractory artery occlusions?. AJNR Am J Neuroradiol. 2015. 36: 552-6
5. Kulcsár Z, Marosfoi M, Berentei Z, Szikora I. Continuous thrombolysis and repeated thrombectomy with the Penumbra System™ in a child with hemorrhagic sinus thrombosis: Technical note. Acta Neurochir (Wien). 2010. 152: 911-6
6. Mortimer AM, Bradley MD, O’Leary S, Renowden SA. Endovascular treatment of children with cerebral venous sinus thrombosis: A case series. Pediatric Neurol. 2013. 49: 305-12
7. Ramos SK, Phillips J, Lin J, Moresco K, Meagher S. Successful rheolytic mechanical thrombectomy of cerebral venous thrombosis in a pediatric patient. J Neurosurg Pediatr. 2013. 11: 140-3
8. Wasay M, Bakshi R, Dai A, Roach S. Local thrombolytic treatment of cerebral venous thrombosis in three paediatric patients. J Pak Med Assoc. 2006. 56: 555-6
9. Waugh J, Plumb P, Rollins N, Dowling MM. Prolonged direct catheter thrombolysis of cerebral venous sinus thrombosis in children: A case series. J Child Neurol. 2012. 27: 337-45
10. Zhang A, Collinson RL, Hurst RW, Weigele JB. Rheolytic thrombectomy for cerebral sinus thrombosis. Neurocrit Care. 2008. 9: 17-26