- Department of Neurosurgery, Fukuoka University Hospital and School of Medicine, 7-45-1 Nanakuma Jonan-Ku, Fukuoka, Japan.
DOI:10.25259/SNI_42_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yoshinobu Horio, Kenji Fukuda, Koichi Miki, Noriko Hirao, Mitsutoshi Iwaasa, Hiroshi Abe, Tooru Inoue. Dynamic assessment of internal carotid artery and elongated styloid process in a case of bilateral carotid artery dissection. 27-Jun-2020;11:163
How to cite this URL: Yoshinobu Horio, Kenji Fukuda, Koichi Miki, Noriko Hirao, Mitsutoshi Iwaasa, Hiroshi Abe, Tooru Inoue. Dynamic assessment of internal carotid artery and elongated styloid process in a case of bilateral carotid artery dissection. 27-Jun-2020;11:163. Available from: https://surgicalneurologyint.com/surgicalint-articles/10099/
Abstract
Background: Vascular Eagle syndrome is that an elongated styloid process causes ischemic stroke due to internal carotid artery (ICA) dissection. Dynamic assessment using radiological imaging has not been well investigated. We assessed the change in the relative positional relationship between the elongated styloid process and the ICA using a cone-beam computed tomography (CBCT).
Case Description: A 46-year-old female presenting with disturbance of consciousness, right hemiparesis, and aphasia was admitted to our hospital. Initial CT analysis showed a bilateral elongated styloid process. Magnetic resonance angiography (MRA) showed occlusion of the left ICA and a near occlusion of the right ICA. MRA also revealed the intimal flap and intramural hematoma in the bilateral ICA. Digital subtraction angiography showed bilateral ICA occlusion and carotid artery stenting was performed subsequently. After that, we visualized the movement of carotid stent with CBCT fusion methods. The stent moved forward and backward at the attachment point of the styloid process during head rotation, and there was a possibility that mechanical stress was emphasized at this point. Styloidectomy was performed after her rehabilitation. The patient did not experience a recurrence of stroke.
Conclusion: We showed that repeated attachment of the styloid process and ICA may trigger an ICA dissection during head rotation. This finding would be helpful for understanding the causes of vascular Eagle syndrome.
Keywords: Cone-beam computed tomography, Dissection, Eagle syndrome
INTRODUCTION
An elongated styloid process is a major cause of an internal carotid artery (ICA) dissection.[
CASE PRESENTATION
A 46-year-old female presenting with disturbance of consciousness, right hemiparesis, and aphasia was admitted to our hospital. Neurological examinations revealed a National Institutes of Health Stroke Scale score of 31. Initial CT analysis showed a bilateral elongated styloid process (right 31.1 cm and left 33.2 cm). Diffusion-weighted magnetic resonance imaging revealed acute cerebral infarction in the left frontal lobe. Magnetic resonance angiography (MRA) showed occlusion of the left ICA and a near occlusion of the right ICA. MRA also revealed the intimal flap and intramural hematoma in the bilateral ICA [
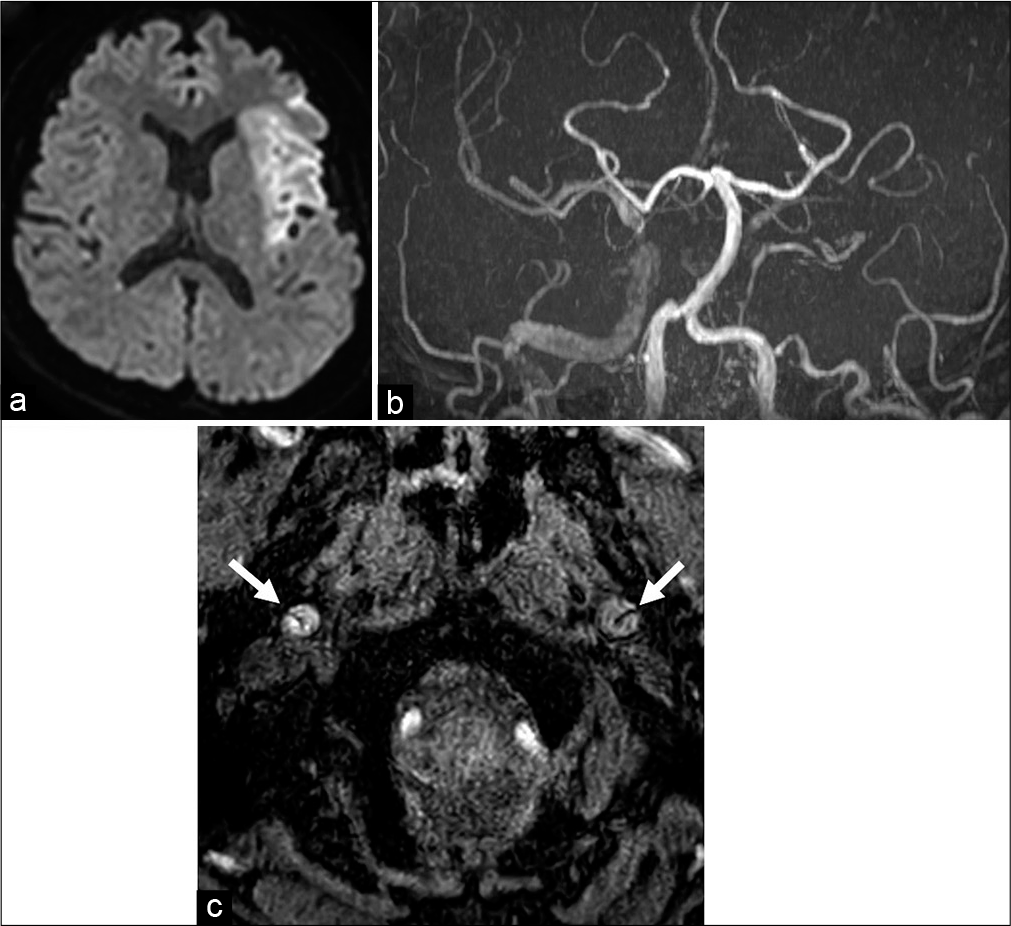
Figure 1:
(a) Initial diffusion-weighted imaging showed a hyperintense area in the left middle cerebral artery (MCA) territory. (b) Magnetic resonance angiography (MRA) showed that the right internal carotid artery (ICA) was nearly occluded and the left ICA was fully occluded. The left distal MCA could not be detected using MRA. (c) The intimal flap (white arrow) was detected in the bilateral cervical ICA.
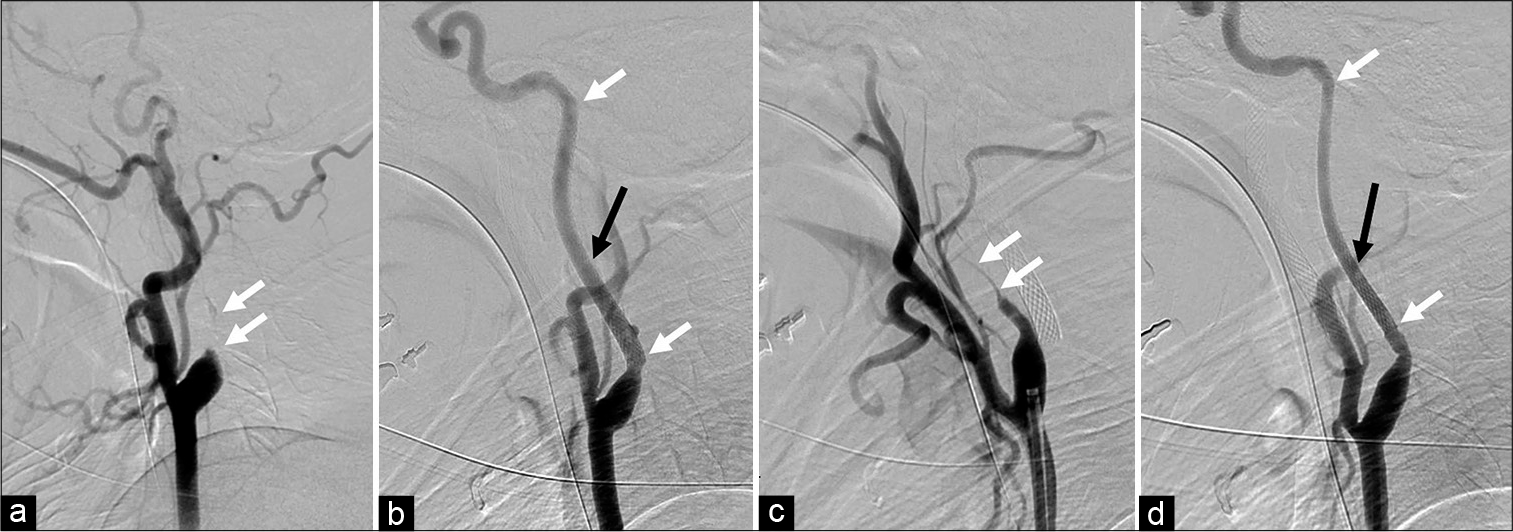
Figure 2:
(a) Digital subtraction angiography showed an occlusion in the cervical portion of the left internal carotid artery (ICA) (white arrow). (b) Carotid artery stenting (CAS) was performed for the left ICA followed by percutaneous transluminal angioplasty (PTA). The proximal and distal edges of the stent are represented with a white arrow. The left styloid process (black arrow) was close to the left ICA. (c) The right ICA was nearly occluded (white arrow). (d) PTA and CAS were also performed on the right ICA. The proximal and distal edges of the stent are represented with white arrows. The right styloid process was also close to the right ICA.
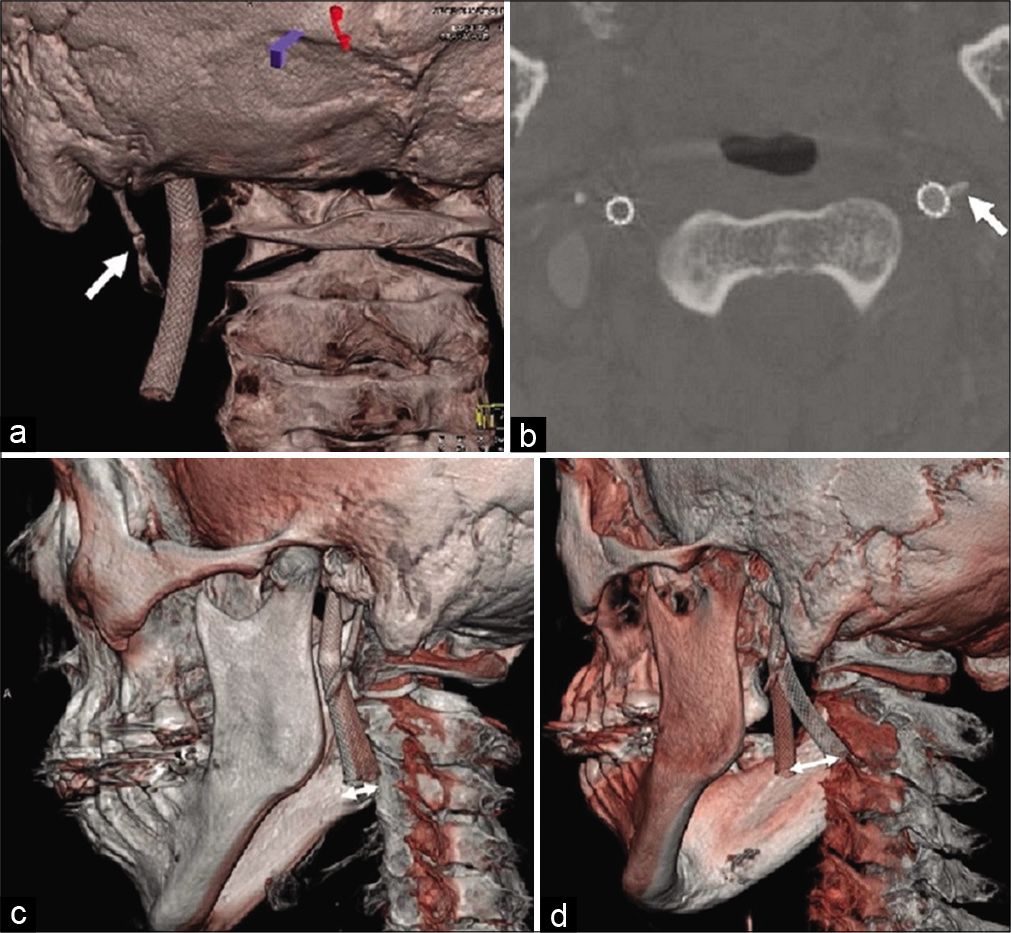
Figure 3:
Cone-beam computed tomography after carotid artery stenting showed that the left carotid stent was close to the elongated styloid process (white arrow) according to the 3D (a) and axial images (b). (c) The fusion image between the neutral position (red skull) and the left rotation (white skull) shows forward movement (white double arrows) of the carotid stent. (d) The movement was more remarkable (white double arrows) in the fusion image between neutral position (red skull) and right rotation (white skull).
DISCUSSION
Eagle syndrome is divided into two main types: classical and vascular Eagle syndrome. Classical Eagle syndrome is characterized by pain in the throat, referred otalgia, and a foreign body sensation in the throat caused by attaching the lower cranial nerves with the elongated styloid process.[
Bilateral ICA dissection due to elongated styloid process is rare and only five cases have been previously reported.[
CONCLUSION
We used a dynamic assessment with CBCT to analyze the styloid process and the ICA during ICA dissection. Repeated attachment of the ICA and the styloid process may trigger an ICA dissection during head rotation. This finding would be helpful for understanding the causes of vascular Eagle syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the assistance of editorial services that provided language help.
References
1. David J, Lieb M, Rahimi SA. Stylocarotid artery syndrome. J Vasc Surg. 2014. 60: 1661-3
2. Eagle WW. Elongated styloid processes: Report of two cases. Arch Otolaryngol. 1937. 25: 584-7
3. Faivre A, Abdelfettah Z, Rodriguez S, Nicoli F. Neurological picture. Bilateral internal carotid artery dissection due to elongated styloid processes and shaking dancing. J Neurol Neurosurg Psychiatry. 2009. 80: 1154-5
4. Fukuda K, Higashi T, Okawa M, Iwaasa M, Abe H, Inoue T. Fusion technique using three-dimensional digital subtraction angiography in the evaluation of complex cerebral and spinal vascular malformations. World Neurosurg. 2016. 85: 353-8
5. Galletta K, Granata F, Longo M, Alafaci C, De Ponte FS, Squillaci D. An unusual internal carotid artery compression as a possible cause of eagle syndrome-a novel hypothesis and an innovative surgical technique. Surg Neurol Int. 2019. 10: 174-
6. Hooker JD, Joyner DA, Farley EP, Khan M. Carotid stent fracture from stylocarotid syndrome. J Radiol Case Rep. 2016. 10: 1-8
7. Ogura T, Mineharu Y, Todo K, Kohara N, Sakai N. Carotid artery dissection caused by an elongated styloid process: Three case reports and review of the literature. NMC Case Rep J. 2015. 2: 21-5
8. Razak A, Short JL, Hussain SI. Carotid artery dissection due to elongated styloid process: A self-stabbing phenomenon. J Neuroimaging. 2014. 24: 298-301
9. Renard D, Azakri S, Arquizan C, Swinnen B, Labauge P, Thijs V. Styloid and hyoid bone proximity is a risk factor for cervical carotid artery dissection. Stroke. 2013. 44: 2475-9
10. Shindo T, Ito M, Matsumoto J, Miki K, Fujihara F, Terasaka S. A case of juvenile stroke due to carotid artery dissection from an elongated styloid process-revisiting conservative management. J Stroke Cerebrovasc Dis. 2019. 28: 104307-
11. Smoot TW, Taha A, Tarlov N, Riebe B. Eagle syndrome: A case report of stylocarotid syndrome with internal carotid artery dissection. Interv Neuroradiol. 2017. 23: 433-6
12. Soo OY, Chan YL, Wong KS. Carotid artery dissection after prolonged head tilting while holding a newborn baby to sleep. Neurology. 2004. 62: 1647-8
13. Sveinsson O, Kostulas N, Herrman L. Internal carotid dissection caused by an elongated styloid process (eagle syndrome). BMJ Case Rep. 2013. 2013: 009878-
14. Torikoshi S, Yamao Y, Ogino E, Taki W, Sunohara T, Nishimura M. A staged therapy for internal carotid artery dissection caused by vascular eagle syndrome. World Neurosurg. 2019. 29: 133-9
15. Zuber M, Meder JF, Mas JL. Carotid artery dissection due to elongated styloid process. Neurology. 1999. 53: 1886-7