- Department of Neurosurgery, University of Calgary, Calgary, Canada.
- Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, Canada.
Correspondence Address:
Yves Starreveld, MD, PhD, FRCSC, Assistant Professor of Neurosurgery, University of Calgary, Department of Clinical Neurosciences, 12th Floor, 1403 – 29th St NW, Calgary, AB, T2N 2T9 Canada.
DOI:10.25259/SNI_1053_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Neilen P Rasiah1, Abdulrahman Albakr1, Suzanne Kosteniuk2, Yves Starreveld1. Early and isolated breast cancer metastasis to the pituitary: A case report and systematic review. 14-Oct-2022;13:462
How to cite this URL: Neilen P Rasiah1, Abdulrahman Albakr1, Suzanne Kosteniuk2, Yves Starreveld1. Early and isolated breast cancer metastasis to the pituitary: A case report and systematic review. 14-Oct-2022;13:462. Available from: https://surgicalneurologyint.com/surgicalint-articles/11936/
Abstract
Background: Pituitary metastases (PMs) arising from breast cancer tend to occur many years following initial diagnosis, and after other systemic metastasis have been identified. Survival is generally considered to be poor. However, there are cases where patients present with an isolated metastatic lesion in the pituitary. Survival in this subset of patients has not been evaluated. We present a case of isolated PM that presented two years after initial diagnosis of breast cancer. We performed a systematic review of 38 breast cancer patients with PM. We report presentation, treatment strategy, and outcomes of breast cancer metastasis to the pituitary and highlight cases of isolated PM.
Case Description: A 39 year old female presented with complaints of headache and polydipsia two years after diagnosis with breast cancer. Systemic workup was unremarkable, but brain imaging identified an isolated PM. Transsphenoidal debulking was performed with adjuvant radiation therapy (RT) targeted to the sellar region. Unfortunately, she passed away 9 months later from systemic progression.
Conclusion: A total of 38 patients were included systematic review. Of these, 13 had isolated PM. Prevalent signs/ symptoms included visual disturbance, diabetes insipidus (DI), and hypothalamic dysfunction. Patients treated with surgical resection and adjuvant chemotherapy (ChT), or RT had better survival than those treated with resection alone. Patients that receive treatment for isolated PM may survive for many years without progression or recurrence.
Keywords: Breast cancer, Breast neoplasms, Pituitary gland, Pituitary metastasis, Pituitary neoplasms, Skull base
INTRODUCTION
Pituitary metastasis (PM) was first described by Ludwig Benjamin in 1857 and subsequently by Harvey Cushing in 1913. [
PM most commonly arises from breast cancer.[
Here, we present the case of a patient with a solitary metastatic lesion at the pituitary gland detected only 2 years after diagnosis of invasive ductal carcinoma. A systematic review was performed with the aim of quantifying presentation and treatment outcomes in patients with PM stemming from breast cancer, which highlights cases where patients had isolated PM.
CASE REPORT
A 39-year-old woman was diagnosed with invasive ductal carcinoma in August 2018. She completed neoadjuvant therapy and then underwent total mastectomy in January 2019. The pathology report described invasive ductal carcinoma with marked treatment effect, in a background of extensive ductal carcinoma in situ. The tumor was immunopositive for estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2) and immunonegative for progesterone receptor (PR). The patient was seen in clinic in July 2020 with new headache, unquenchable thirst, and diminished visual acuity of the left eye. A magnetic resonance image (MRI) demonstrated a 1.6 × 1.7 × 2.5 cm lesion on the pituitary stalk [
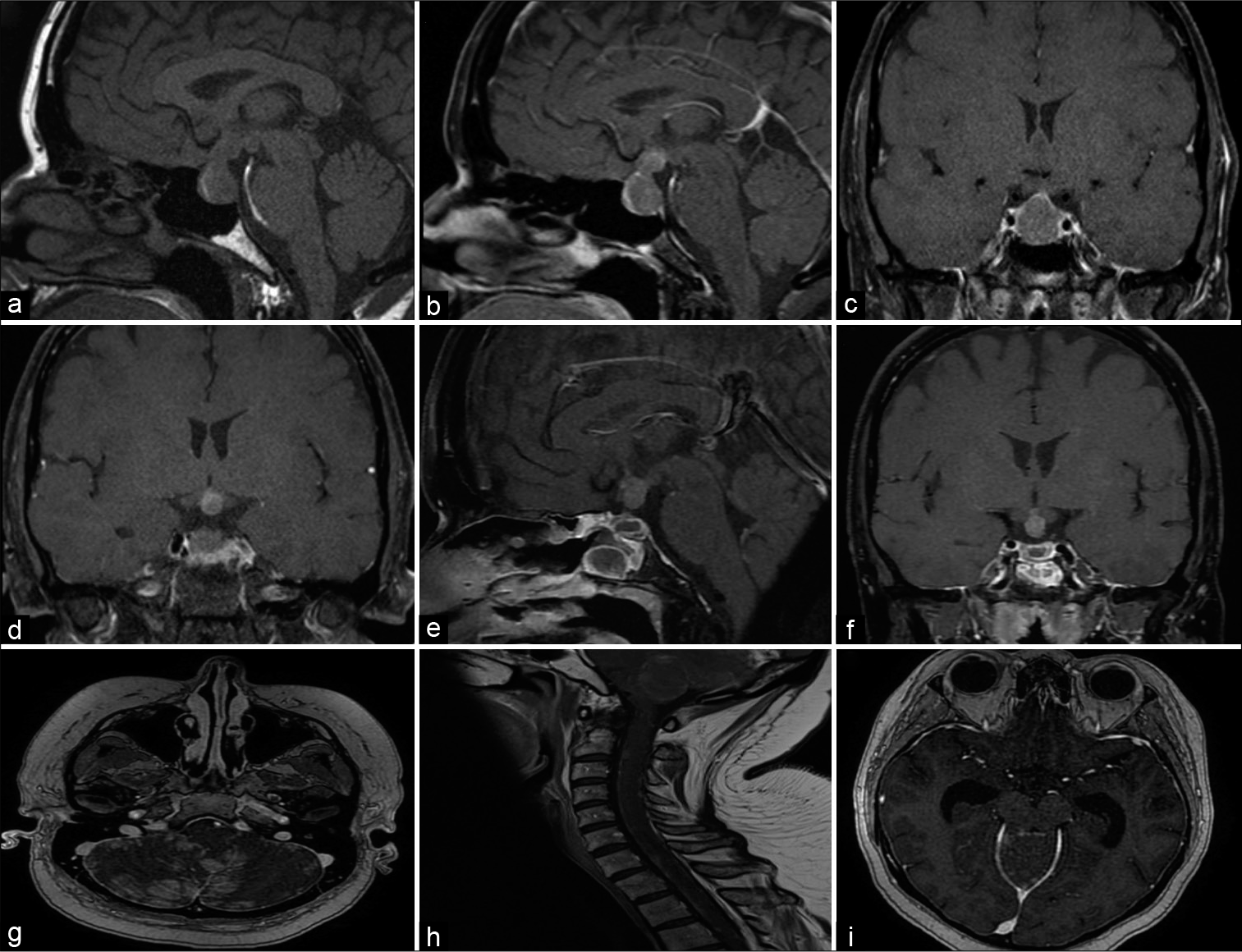
Figure 1:
MRI showing PM and. (a-d) Preoperative coronal and sagittal T1 MRI with and without contrast showing a lesion in the pituitary stalk and gland. There is mild splaying of the optic chiasm with lateral displacement of the left optic nerve. (e and f) A residual tumor involving the stalk and hypothalamus was identified on postoperative coronal and sagittal T1 MRI with contrast. (g and h) A follow-up MRI of the brain and cervical spine with contrast showed a leptomeningeal metastatic disease. (i) A follow-up MRI of the brain with contrast showing hydrocephalus due to leptomeningeal disease. A palliative ventriculoperitoneal shunt was placed.
The patient was seen for follow-up in December 2020. She complained of bowel and bladder incontinence, which prompted MRI of the head and spine. While her residual tumor involving the stalk and hypothalamus had diminished in size, multiple intracranial areas of abnormal enhancement involving the cerebellum, brainstem, and supratentorial sulci were present [
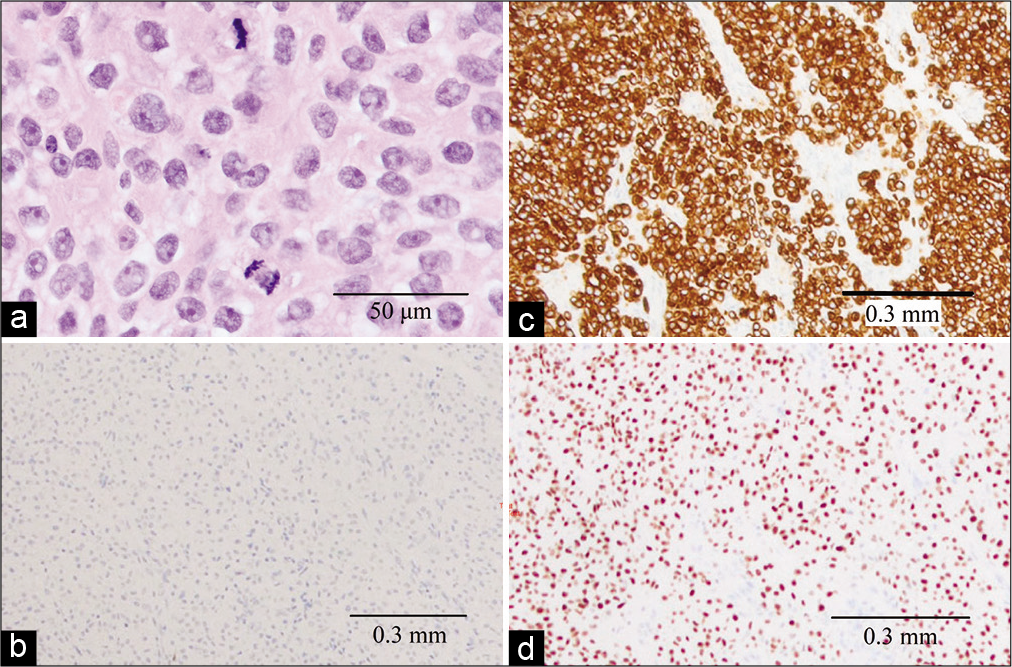
Figure 2:
Histological appraisal of PM. (a) Hematoxylin and eosin stained section demonstrating neoplastic cells with anaplastic nuclei, prominent nucleoli, and mitotic activity. (b) Cytokeratin 7 immunohistochemically stained section showing strong, diffuse cytoplasmic immunopositivity. (c) Cytokeratin 20 immunohistochemically stained section showing immunonegativity. (d) Gata3 labeled section showing strong nuclear positivity.
LITERATURE REVIEW
Methods
Search strategy
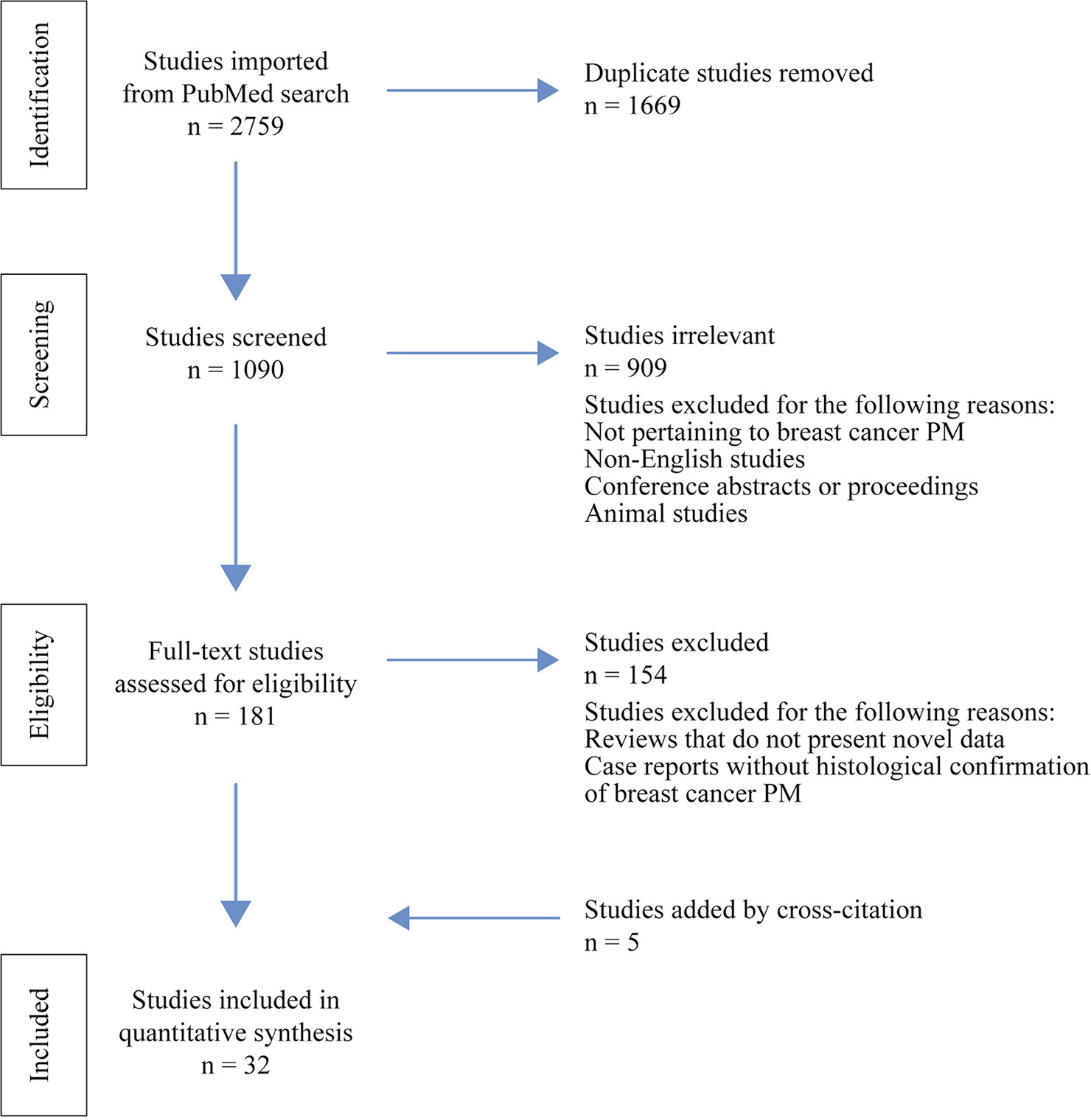
We aimed to ascertain all peer-reviewed publications reporting PM arising from a primary breast cancer lesion. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was employed to ensure quality, transparency, and completeness of our study.[
Papers were collected through Ovid MEDLINE in February 2021. The search results are summarized in [
Data extraction
Data collected included: breast cancer subtype and histological features, clinical presentation, age at PM diagnosis, time between breast cancer diagnosis and PM, gender, hormonal profile, follow-up details, treatment modalities, disease progression, and overall survival. In cases where patients had breast cancer relapses, the time from first diagnosis to PM was reported. All endocrine diagnoses and laboratory values were reported before surgical resection of PM. Resections were considered to be total resections unless specifically stated otherwise (partial resection or debulking). Descriptive statistics were presented for variables and expressed as mean ± S.E.M.
RESULTS
Patient characteristics and presentation
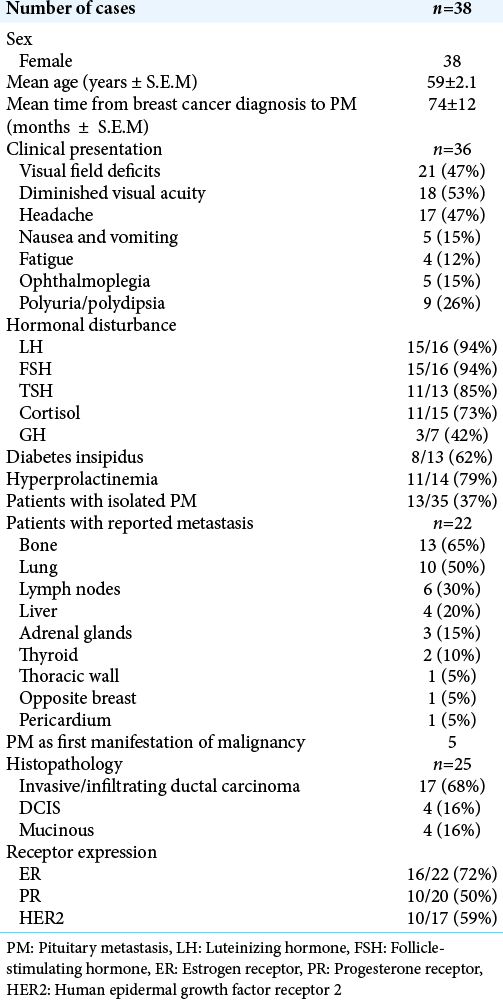
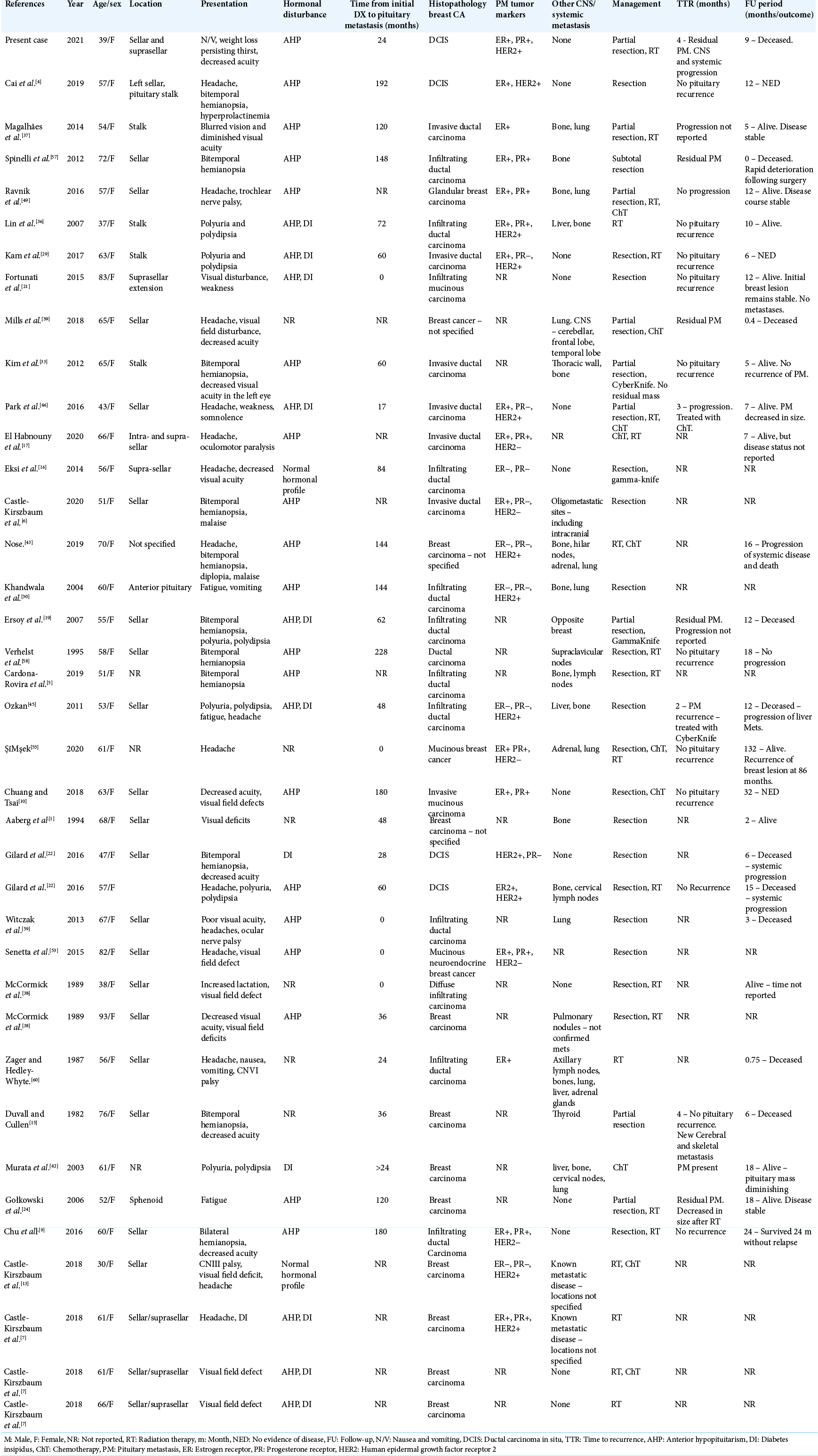
Our literature search identified 32 studies with histologically confirmed PM from breast cancer. Including our patient, 38 patients were included in the analysis. The data are summarized in
Visual field deficits and decreased visual acuity were the most common presentations, occurring in 21 and 16 patients, respectively. Polyuria or polydipsia was reported in nine patients, and DI was confirmed in six of nine patients with available data. Other common symptoms included headache (17), nausea and vomiting (5), and fatigue (4). There were three patients whose only presenting complaints were headache, nausea, or fatigue. Ophthalmoplegia was reported in six patients. Anterior pituitary function was disrupted in all but three cases with available hormonal data (29/32). The gonadal hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were most affected, and hyposecretion was noted in 15 of 16 cases that reported hormonal values. We assessed triiodothyronine (T3), thyroxine (T4), thyroid stimulating hormone (TSH). Hypothyroidism was noted in 8 of 11 patients with reported values for TSH and thyroid hormones. One patient had primary hypothyroidism before PM diagnosis which was not included. Hypocortisolism was reported in 10 of 15 cases that assessed function of the hypothalamic-pituitary-adrenal axis. Hyperprolactinemia was found in nine of 15 cases that reported prolactin levels.
Additional sites of metastasis
The majority of patients had additional metastatic sites at the time of PM diagnosis. Data on burden of metastatic disease were available in 35 cases, and 22 were found to have additional metastatic lesions at the time of PM diagnosis. Metastasis to bone and lung were the most common, occurring in 13 and 10 patients, respectively. Other sites included lymph nodes (6), liver (4), adrenal gland (3), thyroid (2), thoracic wall (1), and opposite breast (1). There were only three cases that reported central metastasis in addition to PM. Of the nine patients diagnosed with PM within 24 months of initial breast cancer diagnosis, four had additional systemic metastases.
Histological features
The majority of PMs were reported from primary invasive ductal carcinoma (17/25 cases that specified histological class). Notably, there were four cases of mucinous carcinoma, a relatively rare type of breast cancer. We then quantified the prevalence of hormonal receptor expression in PM. Expression profiles of ER, PR, and HER2 has prognostic implications and help guide treatment in patients with breast cancer.[
Treatment and outcomes
Follow-up was available for 27 of 38 cases at a mean of 15 months after PM diagnosis [
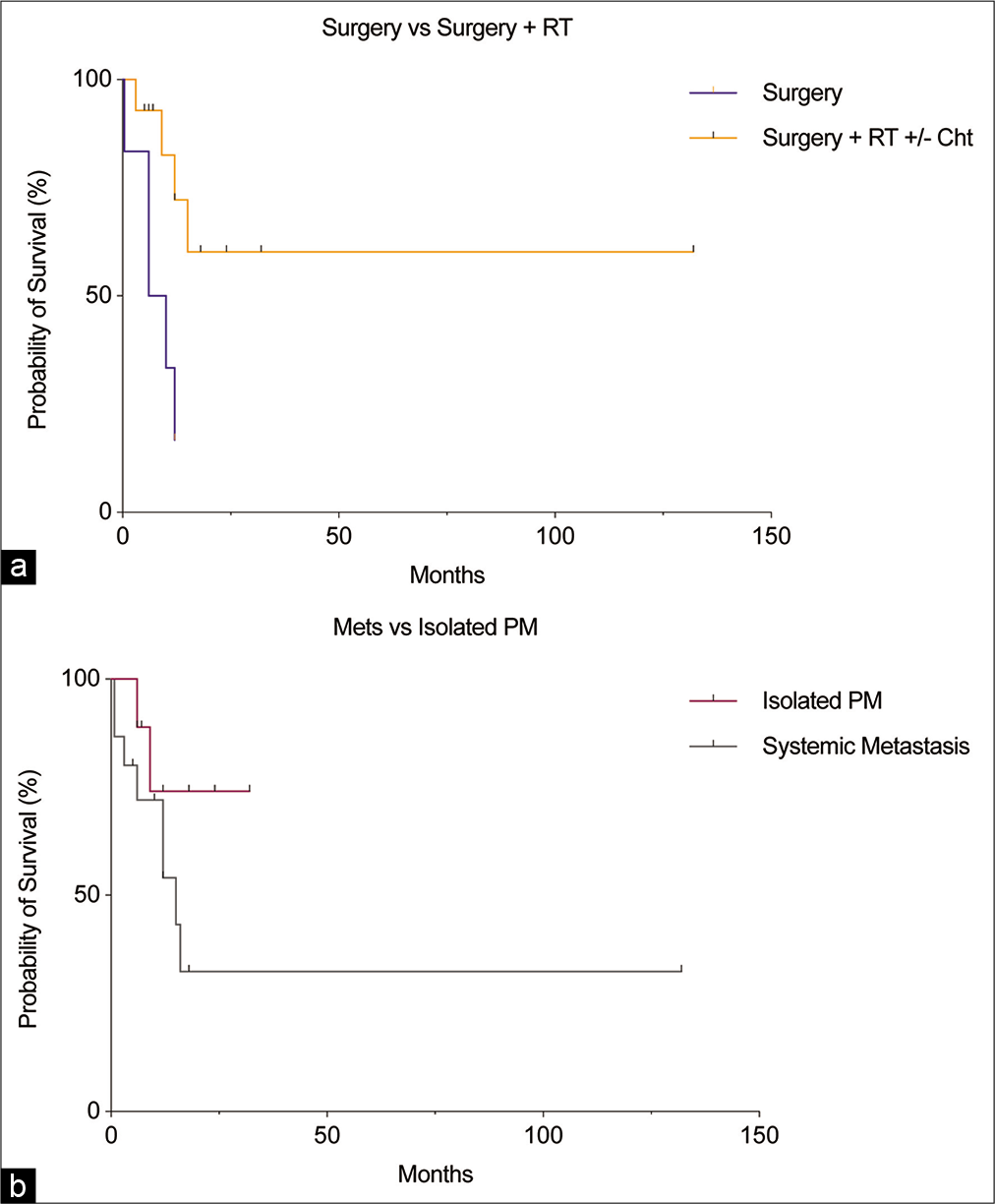
Assessing the effectiveness of treatments targeted to PM can be confounded by systemic disease. Patients with isolated PM had better outcomes compared to those with systemic metastases [
DISCUSSION
Our review included data from 38 patients with confirmed breast cancer metastasis to the pituitary. The most common primary tumor was invasive ductal carcinoma. Interestingly, there was a high proportion of mucinous breast cancers (16%), which is higher than the 2–3% reported among all breast cancers.[
Metastases to the pituitary may present insidiously, and many are found incidentally on autopsy.[
Hormonal disturbance is a common complication in PM. Particularly, DI has been reported in up to 70% of symptomatic PM.[
Breast cancer is the most common malignancy in women and is a frequent source of PM.[
Breast cancers that express HER2 are more aggressive and frequently spread to the CNS. In our study, HER2 was positive in 10/17 (58%) of cases that reported HER2 expression in the primary breast lesion. This is higher than the reported prevalence of HER2 positivity in primary breast cancers of 15–30%,[
There was a trend toward improved local control and survival in patients treated with adjuvant RT. This was particularly true following partial resection. There was only one case of PM recurrence, which followed total resection in a patient that did not receive adjuvant therapy. In a large cohort of patients with PM, 76% received RT (whole-brain radiotherapy or local RT), 21% received surgery, and within the surgical group, the majority also received neoadjuvant or adjuvant RT. There was an association between surgical resection and prolonged survival; however, this may be due to selection of patients with severe symptoms and good functional status.[
New strategies for treating cancer metastasis to the CNS are being investigated. Various immune checkpoint inhibitors (ICIs) circumvent the malignant cell’s immune evasive mechanisms[
CONCLUSION
Several patients in our analysis were diagnosed with isolated PM, and many survived without progression or recurrence for years after treatment. The presence of additional metastatic sites has the largest influence on survival. Patients that underwent resection combined with adjuvant ChT + RT or RT alone, whether they had isolated PM or multiple metastatic sites, had better survival than those with resection alone. Therefore, the data suggest that surgical resection combined with RT.
Limitations
The strength of conclusions is limited by the small number of cases. Furthermore, case reports are typically composed to reveal remarkable occurrences aimed to inform on a particular disease phenomena and may not necessarily reflect the typical sequelae in the natural history of a disease. Therefore, as case reports were used to compile the data in our analysis, the sample population may not be entirely representative. However, age at diagnosis, survival, and relative frequency of metastasis to different sites were all similar to that reported from larger studies. Another limitation is the lack of standardization among case reports. Subsequently, we could only quantify parameters based on the available data presented in each report. We also acknowledge that our findings regarding survival following surgical resection with or without adjuvant therapies may be subject to bias as patients who are selected for surgery are healthier with good functional status.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY TABLE
Acknowledgment
The authors would like to thank Ms. Nicole Dunnewold for her assistance in constructing the search strategy.
References
1. Aaberg TM, Kay M, Sternau L. Metastatic tumors to the pituitary. Am J Ophthalmol. 1995. 119: 779-85
2. Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004. 13: 1128-35
3. Benjamin C, Ashayeri K, Golfinos JG, Placantonakis DG, Silverman J, Kondziolka D. Treatment of sellar metastases with gamma knife radiosurgery in patients with advanced cancer. Pituitary. 2020. 23: 665-71
4. Cai H, Liu W, Feng T, Li Z, Liu Y. Clinical presentation and pathologic characteristics of pituitary metastasis from breast carcinoma: Cases and a systematic review of the literature. World Neurosurg. 2019. 124: 445-51.e2
5. Cardona-Rovira MG, Casañ-Fernández R, Sanz-Gallur J, Almonacid-Folch E, Nadal-Máñez A. Pituitary metastasis of solid tumors: 2 cases of different presentation. Endocrinol Diabetes Nutr (Engl Ed). 2019. 66: 202-3
6. Castle-Kirszbaum M, Beng Phung T, Luen SJ, Rimmer J, Chandra RV, Goldschlager T. A pituitary metastasis, an adenoma and potential hypophysitis: A case report of tumour to tumour metastasis in the pituitary. J Clin Neurosci. 2020. 81: 161-6
7. Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, King J. Twelve cases of pituitary metastasis: A case series and review of the literature. Pituitary. 2018. 21: 463-73
8. Chiang MF, Brock M, Patt S. Pituitary metastases. Neurochirurgia (Stuttg). 1990. 33: 127-31
9. Chu TP, Tsai CC, Chan WC, Tzen CY, Chang YC. Solitary pituitary metastasis from breast cancer that presented as visual field defect. J Cancer Res Pract. 2016. 3: 140-3
10. Chuang YC, Tsai CC. Pituitary metastasis of breast cancer: A case report. Int J Gerontol. 2018. 12: 267-70
11. Cironi KA, Decater T, Iwanaga J, Dumont AS, Tubbs RS. Arterial supply to the pituitary gland: A comprehensive review. World Neurosurg. 2020. 142: 206-11
12. Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003. 24: 1-27
13. da Silva PL, do Amaral VC, Gabrielli V, Montt Guevara MM, Mannella P, Baracat EC. Prolactin promotes breast cancer cell migration through actin cytoskeleton remodeling. Front Endocrinol (Lausanne). 2015. 6: 186
14. Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007. 13: 3942-50
15. Duvall J, Cullen JF. Metastatic disease in the pituitary: Clinical features. Trans Ophthalmol Soc U K 1962. 1982. 102: 481-6
16. Eksi M, Hasanov T, Yilmaz B, Akakin A, Bayri Y, Bozkurt SU. Isolated metastasis of breast cancer to the pituitary gland. Neurol India. 2014. 62: 70
17. El Habnouny J, Jandou I, Latrech H, Bourgon C. Pituitary metastasis of a breast ductal adenocarcinoma. Ann Med Surg (Lond). 2020. 60: 380-3
18. Engel RH, Kaklamani VG. HER2-positive breast cancer: Current and future treatment strategies. Drugs. 2007. 67: 1329-41
19. Ersoy R, Topaloglu O, Aydin C, Dirikoc A, Cakir B. Pituitary metastasis of breast cancer confirmed by fluorine-18 fluorodeoxyglucose positron emission tomography: A case report. J Endocrinol Invest. 2007. 30: 532-3
20. Fassett D, Couldwell W. Metastases to the pituitary gland. Neurosurg Focus. 2004. 16: E8
21. Fortunati N, Felicetti F, Donadio M, Grossi E, Michelon F, Ritorto G. Pituitary lesions in breast cancer patients: A report of three cases. Oncol Lett. 2015. 9: 2762-6
22. Gilard V, Alexandru C, Proust F, Derrey S, Hannequin P, Langlois O. Pituitary metastasis: Is there still a place for neurosurgical treatment?. J Neurooncol. 2016. 126: 219-24
23. Giuffrida G, Ferraù F, Alessi Y, Cannavò S. Shrinkage of a pituitary metastasis of melanoma induced by pembrolizumab: A case report. J Med Case Rep. 2021. 15: 555
24. Gołkowski F, Trofimiuk M, Czepko R, Buziak-Bereza M, Lopatka P, Adamek D. Two rare cases of pituitary metastases from breast and kidney cancers. Exp Clin Endocrinol Diabetes. 2007. 115: 537-40
25. Javanbakht A, D’Apuzzo M, Badie B, Salehian B. Pituitary metastasis: A rare condition. Endocr Connect. 2018. 7: 1049-57
26. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E. Cancer statistics, 2004. CA Cancer J Clin. 2004. 54: 8-29
27. Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993. 7: 678-86
28. Kabel AM. Tumor markers of breast cancer: New prospectives. J Oncol Sci. 2017. 3: 5-11
29. Kam J, Kam J, Mann GB, Phillips C, Wentworth JM, King J. Solitary pituitary metastasis from HER2-positive breast cancer. Asia Pac J Clin Oncol. 2017. 13: e181-4
30. Khandwala HM, Mirchandani D, Chibbar R, Chow V. Uncommon presentations of cancer patients: CASE 1. Metastatic breast cancer presenting with panhypopituitarism. J Clin Oncol. 2004. 22: 4851-3
31. Kiecker C. The origins of the circumventricular organs. J Anat. 2018. 232: 540-53
32. Kim JS, Kim IA. Evolving treatment strategies of brain metastases from breast cancer: Current status and future direction. Ther Adv Med Oncol. 2020. 12: 1758835920936117
33. Kim YH, Lee BJ, Lee KJ, Cho JH. A case of pituitary metastasis from breast cancer that presented as left visual disturbance. J Korean Neurosurg Soc. 2012. 51: 94
34. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab. 2004. 89: 574-80
35. Kwok CW, Treeck O, Buchholz S, Seitz S, Ortmann O, Engel JB. Receptors for luteinizing hormone-releasing hormone (GnRH) as therapeutic targets in triple negative breast cancers (TNBC). Target Oncol. 2015. 10: 365-73
36. Lin CS, Lin SH, Chiang YH, Sheu LF, Chao TY. Diabetes insipidus revealing an isolated pituitary stalk metastasis of breast cancer. Eur J Neurol. 2007. 14: e11-2
37. Magalhães JF, Bacchin RP, Costa PS, Alves GM, Filho FF, Stella LC. Breast cancer metastasis to the pituitary gland. Arq Bras Endocrinol Metab. 2014. 58: 869-72
38. McCormick PC, Post KD, Kandji AD, Hays AP. Metastatic carcinoma to the pituitary gland. Br J Neurosurg. 1989. 3: 71-9
39. Mills MT, Wharton SB, Connolly DJ, Mirza S, Sinha S. Pituitary apoplexy secondary to metastatic breast carcinoma into a gonadotroph cell adenoma of the pituitary. Br J Neurosurg. 2018. p. 1-4 ahead of print
40. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010. 8: 336-41
41. Morita S, Miyata S. Different vascular permeability between the sensory and secretory circumventricular organs of adult mouse brain. Cell Tissue Res. 2012. 349: 589-603
42. Murata Y, Ogawa Y, Yokoe I, Kariya S, Morio K, Sasaki T. Pituitary stalk metastasis from breast cancer treated with systemic chemotherapy. Oncol Rep. 2003. 10: 1973-5
43. Nose K, Ogata T, Tsugawa J, Inoue T, Nabeshima K, Tsuboi Y. Pituitary metastasis of breast cancer mimicking IgG4-related hypophysitis. eNeurologicalSci. 2019. 14: 13-5
44. O’Donnell JS, Teng MW, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019. 16: 151-67
45. Ozkan C. Pituitary gland metastasis of breast cancer: A case report. Uluslararasi Hematol Onkol Derg. 2012. 22: 1-4
46. Park Y, Kim H, Kim EH, Suh CO, Lee S. Effective treatment of solitary pituitary metastasis with panhypopituitarism in HER2-positive breast cancer by lapatinib. Cancer Res Treat. 2016. 48: 403-8
47. Pasquier D, Darlix A, Louvel G, Fraisse J, Jacot W, Brain E. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer. 2020. 125: 22-30
48. Patel KR, Zheng J, Tabar V, Cohen MA, Girotra M. Extended survival after surgical resection for pituitary metastases: clinical features, management, and outcomes of metastatic disease to the sella. Oncologist. 2020. 25: e789-97
49. Ravnik J, Smigoc T, Bunc G, Lanisnik B, Ksela U, Ravnik M. Hypophyseal metastases: A report of three cases and literature review. Neurol Neurochir Pol. 2016. 50: 511-6
50. Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015. 66: 111-28
51. Sanchez AM, Flamini MI, Zullino S, Russo E, Giannini A, Mannella P. Regulatory actions of LH and follicle-stimulating hormone on breast cancer cells and mammary tumors in rats. Front Endocrinol (Lausanne). 2018. 9: 239
52. Schill F, Nilsson M, Olsson DS, Ragnarsson O, Berinder K, Engström BE. Pituitary metastases: A nationwide study on current characteristics with special reference to breast cancer. J Clin Endocrinol Metab. 2019. 104: 3379-88
53. Senetta R, Castellano I, Garbossa D, Sapino A, Cassoni P. Pituitary metastasis of an unknown neuroendocrine breast carcinoma mimicking a pituitary adenoma. Pathology. 2013. 45: 422-4
54. Shimon I. Metastatic spread to the pituitary. Neuroendocrinology. 2020. 110: 805-8
55. Şi̇Mşek M. Pituitary gland metastasis of breast cancer: A rare case report and review of the literature. J Oncol Sci. 2020. 6: 127-30
56. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344: 783-92
57. Spinelli GP, Lo Russo G, Miele E, Prinzi N, Tomao F, Antonelli M. Breast cancer metastatic to the pituitary gland: A case report. World J Surg Oncol. 2012. 10: 137
58. Verhelst J, Broucke PV, Dua G, Joosens E, Abs R, Verlooy J. Pituitary metastasis mimicking a pituitary adenoma: A description of two cases. Acta Clin Belg. 1995. 50: 31-5
59. Witczak JK, Davies R, Okosieme OE. An unusual case of pituitary apoplexy. QJM. 2013. 106: 861-3
60. Zager EL, Hedley-Whyte ET. Metastasis within a pituitary adenoma presenting with bilateral abducens palsies: Case report and review of the literature. Neurosurgery. 1987. 21: 383-6