- Department of Neurosurgery, Lillian S. Wells, University of Florida, Florida, United States.
- Department of Neurology, University of Florida, Gainesville, Florida, United States.
- Department of Neurosurgery, Mayo Clinic, Jacksonville, Florida, United States.
DOI:10.25259/SNI_100_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dimitri Laurent1, Olgert Bardhi1, Paul Kubilis1, Brian Corliss1, Stephanie Adamczak1, Ndi Geh1, William Dodd1, Sasha Vaziri1, Katharina Busl2, W. Christopher Fox3. Early chemoprophylaxis for deep venous thrombosis does not increase the risk of hematoma expansion in patients presenting with spontaneous intracerebral hemorrhage. 14-Jun-2021;12:277
How to cite this URL: Dimitri Laurent1, Olgert Bardhi1, Paul Kubilis1, Brian Corliss1, Stephanie Adamczak1, Ndi Geh1, William Dodd1, Sasha Vaziri1, Katharina Busl2, W. Christopher Fox3. Early chemoprophylaxis for deep venous thrombosis does not increase the risk of hematoma expansion in patients presenting with spontaneous intracerebral hemorrhage. 14-Jun-2021;12:277. Available from: https://surgicalneurologyint.com/surgicalint-articles/10890/
Abstract
Background: Spontaneous intracerebral hemorrhage (ICH) is a significant cause of morbidity and mortality worldwide. The development of venous thromboembolism (VTE), including deep venous thrombosis or pulmonary embolism, is correlated with negative outcomes following ICH. Due to the risk of hematoma expansion associated with the use of VTE chemoprophylaxis, there remains significant debate about the optimal timing for its initiation following ICH. We analyzed the risk of early chemoprophylaxis on hematoma expansion following ICH.
Methods: We performed a retrospective analysis of patients presenting with spontaneous ICH at single institution between 2011 and 2018. The rate of hematoma expansion was compared between patients that received early chemoprophylaxis (on admission) and those that received conventional chemoprophylaxis (>24 h).
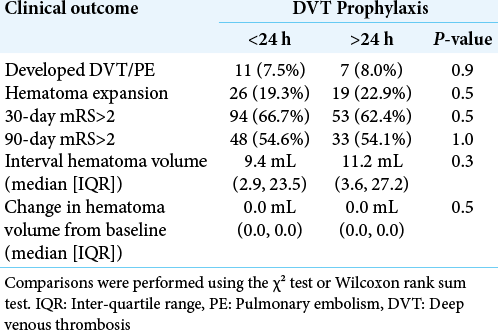
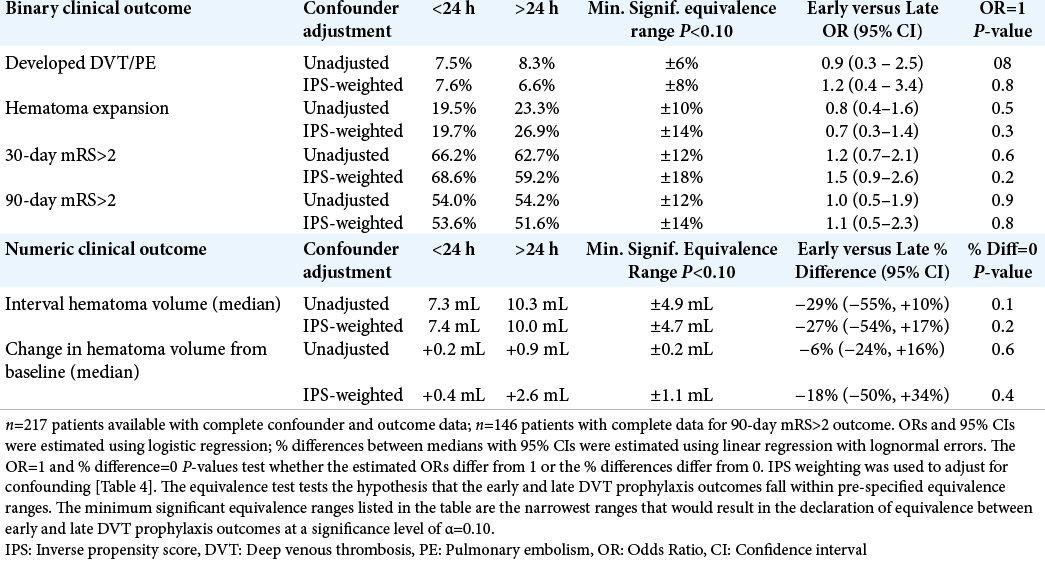
Results: Data for 235 patients were available for analysis. Eleven patients (7.5%) in the early prophylaxis cohort and seven patients (8.0%) in the conventional prophylaxis cohort developed VTE (P = 0.9). Hematoma expansion also did not differ significantly (early 19%, conventional 23%, P = 0.5).
Conclusion: The use of early chemoprophylaxis against venous thromboembolic events following ICH appears safe in our patient population without increasing the risk of hematoma expansion. Given the increased risk of poor outcome in the setting of VTE, early VTE chemoprophylaxis should be considered in patients who present with ICH. Larger, prospective, and randomized studies are necessary to better elucidate the risk of early chemoprophylaxis and potential reduction in venous thromboembolic events.
Keywords: Intracerebral hemorrhage, Deep venous thrombosis, Hematoma expansion, pulmonary embolism, Venous thromboembolism
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) is a significant cause of morbidity and mortality worldwide, with an estimated annual incidence of 300,000–600,000/year in the United States alone.[
Preventive strategies to reduce the incidence of VTE include early mobilization, the use of intermittent lower extremity mechanical compression devices, and chemoprophylaxis in the form of unfractionated heparin (UFH) or low-molecular weight heparin (LMWH).[
Given the uncertainty of these recommendations pertaining to the optimal time point of initiation of chemical VTE prophylaxis, there is marked variability in practice. In our neurosurgical practice, we adapted a practice standardizing early initiation of chemical DVT prophylaxis, often at the time of admission. This practice is done in an attempt to mitigate the negative effect of VTE on outcomes in patients suffering from ICH. At present, there is no great evidence on the safety profile of early administration of VTE in ICH patients. In this study, we report our findings of this practice. We hypothesized that the risk of hemorrhagic expansion is low, and outweighed by the possible benefit of reducing the incidence of VTE. The aims of this study were threefold: (1) determine the safety profile of early VTE prophylaxis in patients who present to the hospital with spontaneous ICH; (2) determine the risk of hematoma expansion in early implementation of LMWH/UFH as compared to delayed administration; and (3) determine the difference in incidence of DVT/PE in those patients who received early LMWH/UFH as compared to delayed administration.
MATERIALS AND METHODS
Patient selection
The University of Florida Institutional Review Board (IRB) approved the study protocol before patient enrollment (IRB# 201801414). We queried the hospital billing database to identify patients who were admitted with non-traumatic ICH (ICD I61) between January 1, 2011, and December 31, 2018. Adult patients (age 18 and older) who had been treated at our institution for spontaneous ICH during the study timeframe were retrospectively enrolled under a full waiver of informed consent. Patients were excluded if they had no interval computed tomography (CT) scan available for review, or underwent limitations of care including comfort measures on initial evaluation.
Data acquisition
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Florida.[
Early initiation of chemoprophylaxis was defined as initiation on admission to our institution, with the first dose administered within 24 h of admission. VTE chemoprophylaxis was considered “conventional” if the first dose was administered 24–72 h after admission. Hematoma volumes were calculated based on CT scans using the ABC/2 method.[
Statistical analysis
We used the χ2 test or Fisher exact test to perform univariate comparisons of proportions between early and late VTE prophylaxis cohorts. We used the Wilcoxon rank sum test to test for shift in the early versus late distributions of numeric variables (e.g., initial hematoma volume, and interval hematoma volume). We used logistic regression to estimate odds ratios (ORs) with 90% confidence intervals (CIs) to show the effect of early versus late treatment on binary clinical outcomes. We used linear regression with lognormal errors to estimate the % difference between early versus late median interval hematoma volumes. Within the framework of these outcome models, we carried out TOST equivalence tests to determine if early and late VTE prophylaxis could be declared equivalent within a pre-specified range (e.g., ±5%, ±10%). The equivalence test generates a P-value for the null hypothesis that the early VTE prophylaxis outcomes fall outside a pre-specified range bracketing the late treatment outcomes. P < 0.10 would indicate equivalence between early and late treatment cohorts.
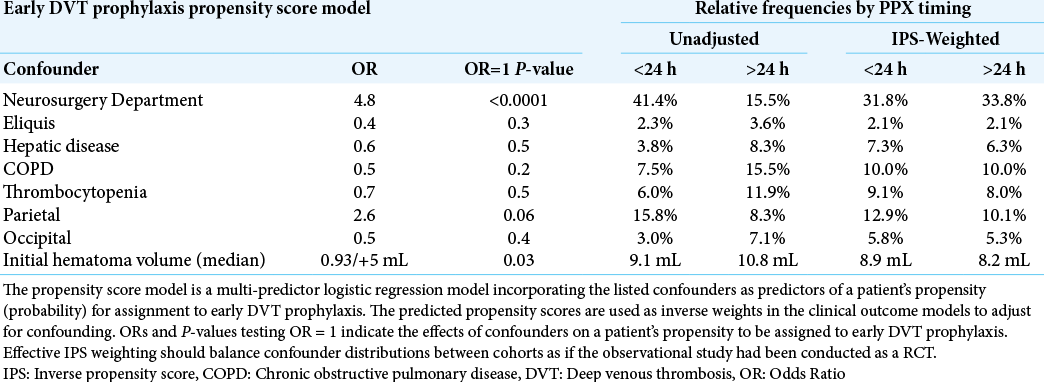
Because of the observational nature of our study, we also considered the possible influence of confounding on equivalence tests and effect size estimates. To avoid over-fitting our outcome models, we chose to adjust for confounding by developing an early VTE prophylaxis propensity score model. The propensity score model is a multi-predictor logistic regression model incorporating relevant confounders as predictors of a patient’s propensity (probability) for assignment to early DVT prophylaxis. The predicted propensity scores are used as inverse weights in the clinical outcome models to adjust for confounding. OR and P-values testing OR=1 in the propensity score model indicate the effects of confounders on a patient’s propensity to be assigned to early DVT prophylaxis. Effective inverse propensity score (IPS) weighting should balance confounder distributions between cohorts as if the observational study had been conducted as a randomized controlled trial (RCT). We initially considered all pre-treatment variables that had a univariate P ≤ 0.5 for difference between early and late treatment cohorts. After initially fitting a logistic regression model containing these confounders, we then excluded predictors from the model with P > 0.5 and refit a final propensity score model. We used the propensity scores from this model as inverse weights in our outcome models, and contrasted unadjusted equivalence tests and estimated ORs with confounder adjusted tests and estimates generated through IPS weighting.
To put negative findings in proper context, we retrospectively calculated the power our study had to declare equivalence between pre-specified ranges, and the power to detect an OR > 1.5 (or < 0.67). We also estimated the sample sizes needed to detect these characteristics with 80% power and two-sided α = 0.10. Finally, we estimated the effect sizes (ORs, % differences) that our study could detect at 80% power. All calculations were performed using SAS Version 9.4 (SAS Institute, Cary NS), R Version 3.5.1 (R Core Team, Vienna, Austria), and PASS Version 16.0.4 (NCSS, LLC. Kaysville, Utah).
RESULTS
Administration of early and late DVT prophylaxis
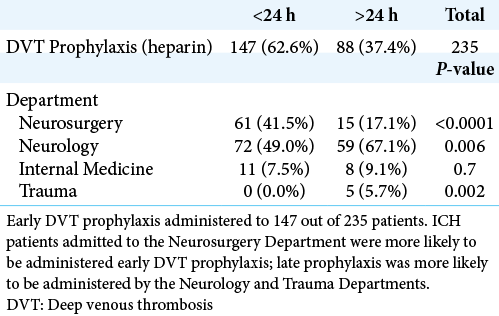
Data for 235 patients were available for analysis. 217 patients had complete confounder and outcome data; and 146 patients had complete data for 90-day mRS > 2.
Of those 235 patients, 62.6% (n = 147) were administered early DVT chemoprophylaxis and 37.4% (n = 88) received conventional chemoprophylaxis [
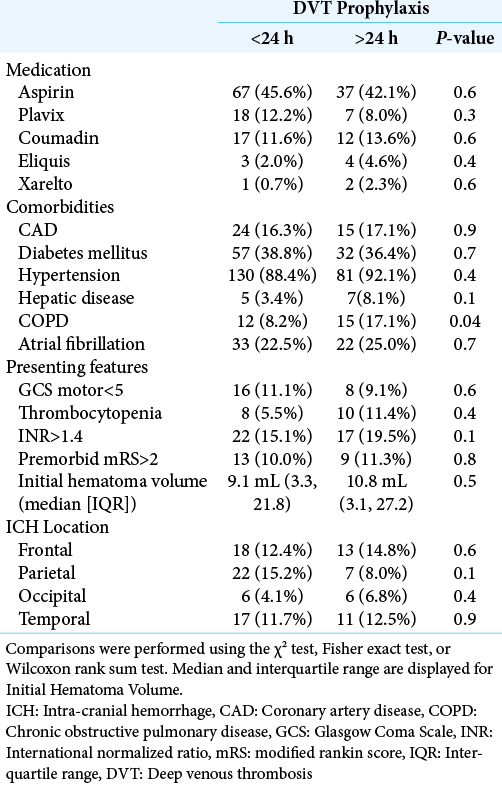
Pre-prophylaxis anticoagulant therapy, comorbidity, presenting features, and ICH location
Univariate comparison of pre-prophylaxis rates of anticoagulant use, comorbidities, presenting features, and ICH location between early and late DVT prophylaxis cohorts is displayed in [
There was no standardized mechanism for obtaining surveillance imaging. Imaging was obtained at the discretion of the treating physician. On average, interval non-contrast CT head was obtained 1.57 days (SD 1.59) after admission, with a range of 1–14 days.
Clinical outcomes – Univariate analysis
Univariate comparisons of the clinical outcomes between early and late DVT prophylaxis cohorts are displayed in [
Clinical outcomes – Adjustment for confounders
We identified the variables in [
[
Consistent with the univariate comparisons from [
Because we observed almost no statistically significant equivalence or effects of early DVT prophylaxis on clinical outcomes relative to late prophylaxis, we retrospectively determined the power our sample sizes had to declare equivalence within the ranges specified in [
These calculations suggest that our study was inadequately powered to declare equivalence between early and late DVT prophylaxis as they influence clinical outcomes. With regard to detecting positive or negative effects of early DVT prophylaxis relative to late treatment, our study had adequate power to detect relatively large effects. However, our study was sufficiently powered to demonstrate equivalence in numeric values (median interval hematoma volume and median change in hematoma volume from baseline). When adjusting for confounders, our study was sufficiently powered to demonstrate equivalence of an interval hematoma volume of 4.7 mL or greater and a change in hematoma volume of within 1.1 mL between patients receiving early and conventional DVT chemoprophylaxis.
DISCUSSION
In the present study, we demonstrated that in this patient population there was no significant risk of early administration of VTE chemoprophylaxis on hematoma expansion following ICH. More specifically, our study was sufficiently powered to suggest equivalence of about one milliliters of volumetric expansion between patients receiving early and conventional VTE prophylaxis. Furthermore, our study was sufficiently powered to suggest total volumetric equivalence of ICH between early and conventional VTE prophylaxis groups within 5 mL. The previous studies have defined hematoma expansion as greater than six milliliters;[
Hemorrhagic stroke is an independent risk factor for the development of venous thromboembolic events, with an estimated incidence of about 2%.[
The present study suggests that early chemoprophylaxis, that is, within 24 h of admission does not result in worse outcomes. Our departmental practice of early chemoprophylaxis is unique even within our institution and was instituted due to the high overall morbidity index in our tertiary care center patient population, as evidenced by a rate of symptomatic thromboembolism at 8%, which is greater than the estimated incidence of 2% patients presenting with hemorrhagic stroke.[
CONCLUSION
The use of early UFH or LMWH for prophylaxis against venous thromboembolic events following ICH appears safe in our patient population without increasing the risk of hematoma expansion. Our study is sufficiently powered to demonstrate equivalence of the risk of hematoma expansion between early and conventional chemoprophylaxis. Larger, prospective, and randomized studies are necessary to better elucidate the risk of early chemoprophylaxis and potential reduction in venous thromboembolic events. This would help clarify the external validity of the results of our single institution, retrospective study.
Limitations
Our study is limited by sample size, precluding our ability to draw conclusions on clinical outcome as related to rate of venous thromboembolic events and functional independence as determined by Modified Rankin Scale. Due to the retrospective nature of this study, there was no standardized mechanism to screen patients for DVT/PE. As such, the incidence of DVT/PE is the result of investigative studies ordered by the treating physician on the suspicion of a thromboembolic event. Clinically silent VTE events are unaccounted for. Furthermore, the retrospective nature of this study does not provide a standardized protocol for surveillance cranial imaging. In an attempt to mitigate biases inherent to the study design, we performed propensity matching; therefore, any effect is unlikely to be related to differences in patient comorbidities between the early and conventional VTE chemoprophylaxis groups.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Kimberly Foli with her help with obtaining IRB approval.
References
1. Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A. Guidelines for the early management of adults with ischemic stroke: A guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The American academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007. 115: e478-534
2. Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA. American society of hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019. 3: 3898-944
3. Andersson T, Söderberg S. Incidence of acute pulmonary embolism, related comorbidities and survival; analysis of a Swedish national cohort. BMC Cardiovasc Disord. 2017. 17: 155
4. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012. 11: 101-18
5. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. Heart disease and stroke statistics-2018 update: A report from the American heart association. Circulation. 2018. 137: e67-492
6. Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014. 71: 158-64
7. 8. Collen JF, Jackson JL, Shorr AF, Moores LK. Prevention of venous thromboembolism in neurosurgery: A metaanalysis. Chest. 2008. 134: 237-49 9. Dennis M, Sandercock P, Reid J, Graham C, Forbes J, Murray G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): A multicentre randomised controlled trial. Lancet (London, England). 2013. 382: 516-24 10. Egleston BL, Uzzo RG, Beck JR, Wong YN. A simple method for evaluating within sample prognostic balance achieved by published comorbidity summary measures. Health Serv Res. 2015. 50: 1179-94 11. Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004. p. 631059-64 12. Gregory PC, Kuhlemeier KV. Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke. Am J Phys Med Rehabil. 2003. 82: 364-9 13. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. 95: 103208 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. 42: 377-81 15. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2015. 46: 2032-60 16. Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: A meta-analysis. Arch Intern Med. 2000. 160: 2327-32 17. Khan NR, Patel PG, Sharpe JP, Lee SL, Sorenson J. Chemical venous thromboembolism prophylaxis in neurosurgical patients: An updated systematic review and meta-analysis. J Neurosurg. 2018. 129: 906-15 18. Kiphuth IC, Staykov D, Kohrmann M, Struffert T, Richter G, Bardutzky J. Early administration of low molecular weight heparin after spontaneous intracerebral hemorrhage. A safety analysis. Cerebrovascular diseases (Basel Switzerland). 2009. 27: 146-150 19. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5 20. Laurent D, Freedman R, Cope L, Sacks P, Abbatematteo J, Kubilis P. Impact of extent of resection on incidence of postoperative complications in patients with glioblastoma. Neurosurgery. 2020. 86: 625-30 21. Nyquist P, Jichici D, Bautista C, Burns J, Chhangani S, DeFilippis M. Prophylaxis of venous thrombosis in neurocritical care patients: an executive summary of evidence-based guidelines: A statement for healthcare professionals from the neurocritical care society and society of critical care medicine. Crit Care Med. 2017. 45: 476-9 22. Ogata T, Yasaka M, Wakugawa Y, Inoue T, Ibayashi S, Okada Y. Deep venous thrombosis after acute intracerebral hemorrhage. J Neurol Sci. 2008. 272: 83-6 23. Paciaroni M, Agnelli G, Venti M, Alberti A, Acciarresi M, Caso V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: A meta-analysis of controlled studies. J Thromb Haemost. 2011. 9: 893-8 24. Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med. 2015. 10: 563-8