- Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Psychiatry, Shourai Hospital, Karatsu, Japan
- Department of Urology, Fukuoka Children’s Hospital, Fukuoka, Japan
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Neurosurgery, Iizuka Hospital, Iizuka, Japan
- Department of Neurosurgery, Hachisuga Hospital, Munakata, Japan
Correspondence Address:
Nobuya Murakami, Department of Neurosurgery, Fukuoka Children’s Hospital, Fukuoka, Japan.
DOI:10.25259/SNI_1089_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nobuya Murakami1, Ai Kurogi1, Satoshi O. Suzuki2, Naoko Akitake3, Takafumi Shimogawa4, Nobutaka Mukae5, Koji Yoshimoto4, Takato Morioka6. Ectopic dorsal root ganglion in cauda equina mimicking schwannoma in a child. 27-Jan-2023;14:33
How to cite this URL: Nobuya Murakami1, Ai Kurogi1, Satoshi O. Suzuki2, Naoko Akitake3, Takafumi Shimogawa4, Nobutaka Mukae5, Koji Yoshimoto4, Takato Morioka6. Ectopic dorsal root ganglion in cauda equina mimicking schwannoma in a child. 27-Jan-2023;14:33. Available from: https://surgicalneurologyint.com/surgicalint-articles/12123/
Abstract
Background: A heterotopic dorsal root ganglion (DRG) is sometimes observed in the vicinity of dysplastic neural structures during surgery for open spinal dysraphism; however, it is rarely associated with closed spinal dysraphism. Distinguish from neoplasms by preoperative imaging study is difficult. Although the embryopathogenesis of a heterotopic DRG has been speculated to be migration disorder of neural crest cells from primary neural tube, its details remain unelucidated.
Case Description: We report a pediatric case with an ectopic DRG in cauda equina associated with a fatty terminal filum and bifid sacrum. The DRG mimicked a schwannoma in the cauda equina on preoperative magnetic resonance imaging. Laminotomy at L3 revealed that the tumor was entangled in the nerve roots, and small parts of the tumor were resected for biopsy. Histopathologically, the tumor consisted of ganglion cells and peripheral nerve fibers. Ki-67 immunopositive cells were observed at the periphery of the ganglion cells. These findings indicate the tumor comprised DRG tissue.
Conclusion: We report detailed neuroradiological, intraoperative and histological findings and discuss the embryopathogenesis of the ectopic DRG. One should be aware of the possibility of ectopic or heterotopic DRGs when cauda equina tumors are observed in pediatric patients with neurulation disorders.
Keywords: Neural crest cell, Neural tube, Neurulation disorder, Fatty terminal filum, Satellite glial cells
INTRODUCTION
A heterotopic dorsal root ganglion (DRG) is sometimes observed in the vicinity of dysplastic neural structures during repair surgery for myelomeningocele, as has been reported in Lendon and Emery’s autopsy study in which 63 of 95 spinal cords from cases with open spinal dysraphism were associated with heterotopic ganglion tissues.[
We treated a pediatric case with an ectopic DRG in cauda equina who had a fatty terminal filum and bifid sacrum. On preoperative magnetic resonance imaging (MRI), the DRG mimicked a schwannoma in the cauda equina. We report detailed neuroradiological, intraoperative, and histological findings and discuss the embryopathogenesis of the lesion.
CASE REPORT
A 4-year- and 6-month-old boy was referred to us due to intermittent dribbling of urine and involuntary loss of a small amount of stool, both of which had been present since birth. No family history was noted. Cognitive development was considered to be appropriate for his age. Neurologically, his anal reflex was weak, but he had no motor dysfunction or sensory disturbance in the lower extremities. He had a tiny dimple at the lumbosacral region. There were no cutaneous stigmata indicative of Café-au-lait spot. His general condition was good, except for short stature which was being followed by a pediatrician.
MRI demonstrated a small tumor in the cauda equina that had isosignal intensity to the spinal cord on three-dimensional T1-weighted imaging (3D-T1WI) and 3D heavily T2WI (3D-hT2WI) and was slightly enhanced with gadolinium [
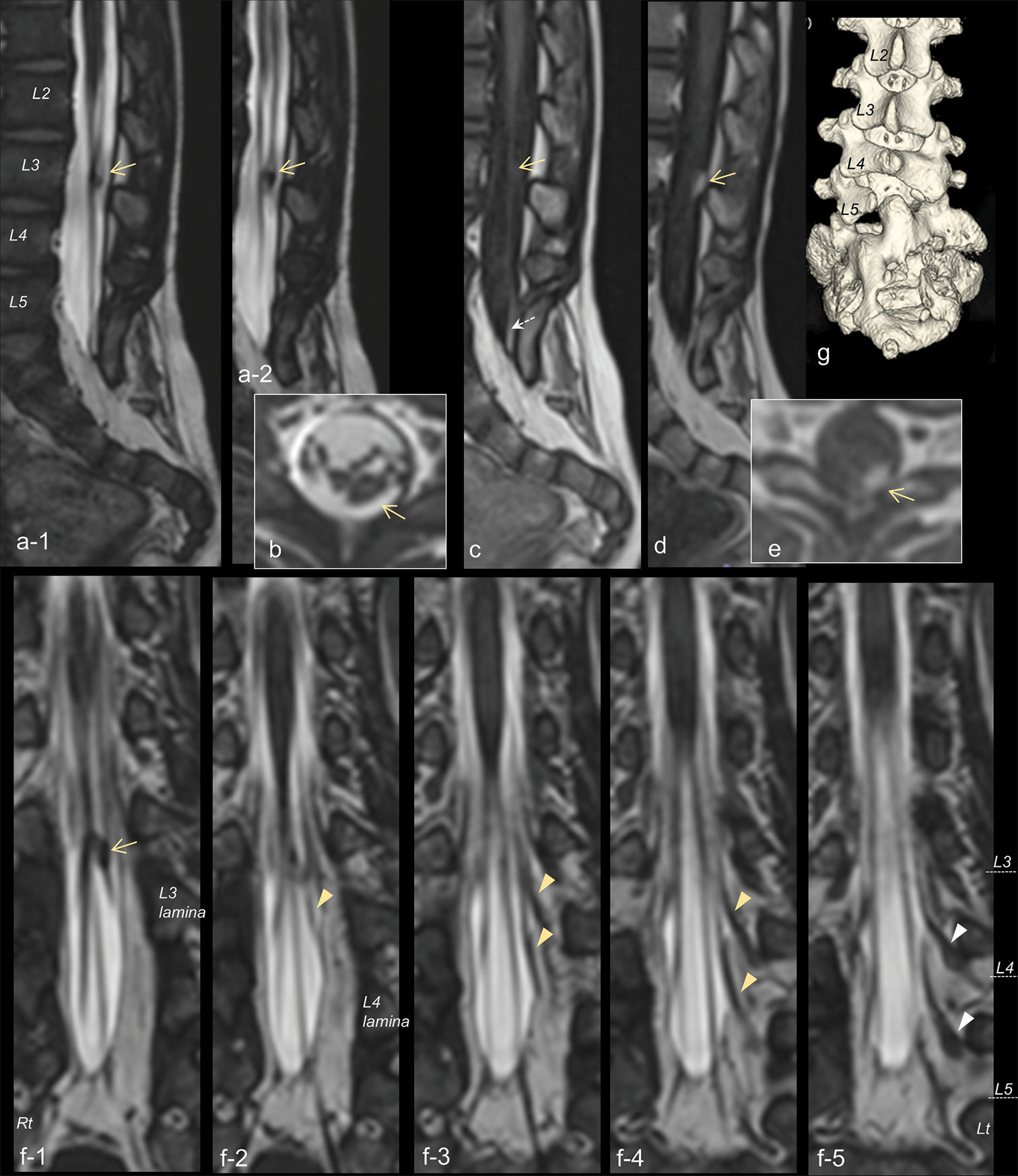
Figure 1:
Sagittal (a-1 and 2) and axial (b) views of three-dimensional heavily T2-weighted imaging (3D-hT2WI) demonstrating a tumor (yellow arrow) in the cauda equina. (c) 3D T1-weighted images (3D-T1WI) showing the isointense lesion (yellow arrow) of the cauda equina and a high intensity lesion (dotted arrow) of the terminal filum. Sagittal (d) and axial (e) views of 3D-T1WI demonstrating the tumor enhanced with gadolinium (yellow arrow). (f-1-5) Serial coronal views of 3D-hT2WI delineating that the tumor (yellow arrow) is located in the dorsal nerve roots (yellow arrowheads) that enter the dural sac at the left L3-L4 and L4-L5 intervertebral space. Normally-positioned dorsal root ganglions (white arrowheads) are observed at the root sleeves as local bulging. The level of each vertebral body is indicated by a dashed line. (g) 3D reconstructions of computed tomography image revealing spina bifida below S2 and abnormal fusion of the sacral lamina of L5-S2.
Although we considered the possibility of a neoplasm, as the patient’s bladder dysfunction had existed since birth and had not progressed, a follow-up MRI was performed 7 months later; this revealed that the cauda equina tumor remained unchanged in size. The diagnosis was uncertain and we proposed diagnostic surgery for the cauda equina tumor, as well as prophylactic untethering of the spinal cord for the fatty terminal filum. Because his family was concerned about postoperative neurological deterioration due to possible damage to the cauda equina, only untethering of the cord was performed at 5 years and 2 months of age.
The fatty filum was severed through L5-S1 interspace. The cauda equina observed in the operative field appeared normal. Histologically, the fatty filum consisted of fibroadipose tissue with a tiny glial fibrillary acidic protein immunopositive tissues. Postoperatively, no de novo neurological abnormalities were observed. His family subsequently provided consent for the patient to undergo surgery for the cauda equina tumor.
Partial removal of the cauda equina tumor was performed at 5 years and 6 months of age. Laminoplastic laminotomy at L3 revealed a spherical tumor with a long axis of 10 mm at the left dorsolateral portion in the dural sac [
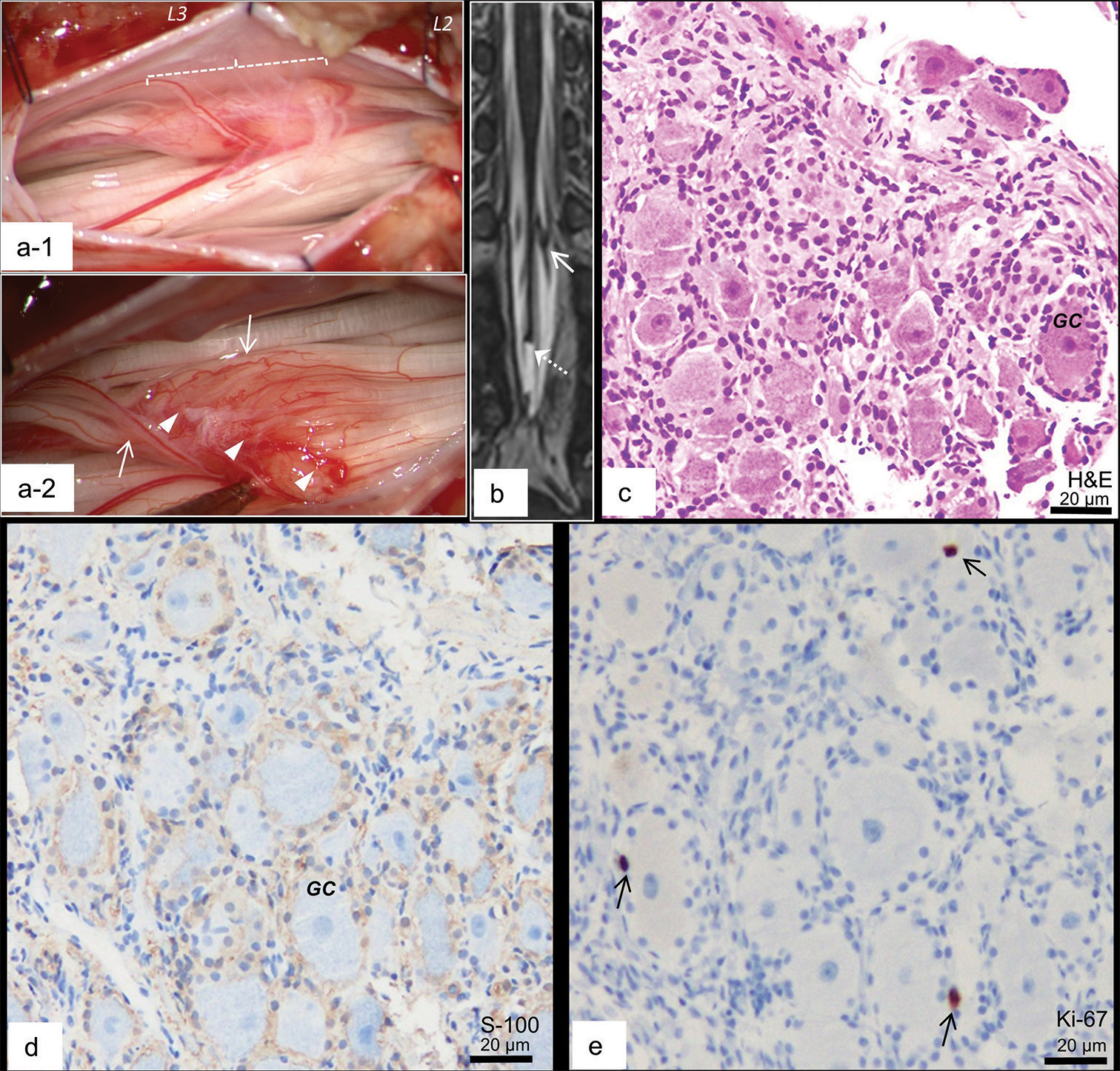
Figure 2:
(a-1 and 2) Microscopic view of the surgery for the cauda equina tumor. Laminotomy at L3 revealed a tumor (a-1; blanket length of 10 mm). (a-2) A few nerve roots running over the lesion surface and extending caudally (arrows); some thinner nerve roots ending at the tumor surface (arrow heads). (b) Coronal view of 3D-hT2WI after the second surgery demonstrated that the cauda equina tumor (arrow) remained unchanged and the untethering of the cord was achieved (dotted arrow). (c-e) Histopathological findings of the resected parts of the tumor stained with hematoxylin and eosin (H & E; c), and immunostained with S-100 (d) and Ki-67 (e). (c) The resected section consisted of ganglion cells (GC) and peripheral nerve fibers. The periphery of the ganglion cells is immunopositive for S-100 (d), a few of which are immunopositive for Ki-67 (arrows; e).
Histopathologically, the resected section consisted of ganglion cells and peripheral nerve fibers [
DISCUSSION
A DRG is derived from neural crest cells located in the dorsal region of the primary neural tube. Some neural crest cells migrate through somite and stop midway lateral to the neural tube, giving rise to the DRG.[
A human DRG normally consists of cell bodies of primary sensory neurons surrounded by satellite glial cells, macrophages, and bundles of peripheral nerve fibers.[
Tumors of the cauda equina in pediatric cases include schwannoma, neurofiboma, myxopapillary ependymoma, dermoid/epidermoid cyst, and paraganglioma.[
CONCLUSION
The present case demonstrate that heterotopic or ectopic DRGs could occur in cauda equina in pediatric patients with neurulation disorders and mimic neoplasms on imaging study. Careful observation of neurological symptoms combined with repeated MRI examination, and diagnostic surgery, when required, is necessary.
Ethics statement
The authors confirm that written informed consent was obtained from the family of the infant described in this report. The authors declare that this work complies with the guidelines for human studies and the research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The Research Foundation of Fukuoka Children’ Hospital.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Conner AK, Fung KM, Peterson JE, Glenn CA, Martin MD. Ectopic ganglion in cauda equina: Case report. J Neurosurg Spine. 2016. 24: 937-40
2. Engelhard HH, Villano JL, Porter KR, Stewart AK, Barua M, Barker FG. Clinical presentation, histology, and treatment in 430 patients with primary tumors of the spinal cord, spinal meninges, or cauda equina. J Neurosurg Spine. 2010. 13: 67-77
3. Haberberger RV, Barry C, Dominguez N, Matusica D. Human dorsal root Ganglia. Front Cell Neurosci. 2019. 13: 271
4. Hamasaki T, Makino K, Morioka M, Hasegawa S, Kurino M, Kuratsu JI. Histological study of paramedian dorsal root ganglia in an infant with split cord malformation. Case report. J Neurosurg. 2006. 104: 415-8
5. Hiraoka A, Morioka T, Murakami N, Suzuki SO, Mizoguchi M. Limited dorsal myeloschisis with no extradural stalk linking to a flat skin lesion: A case report. Childs Nerv Syst. 2018. 34: 2497-501
6. Kalcheim C, Le Douarin NM. Requirement of a neural tube signal for the differentiation of neural crest cells into dorsal root ganglia. Dev Biol. 1986. 116: 451-66
7. Kasemeier-Kulesa JC, Kulesa PM, Lefcort F, Zochodne D. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005. 132: 235-45
8. Krishnan A, Bhavanam S, Zochodne D. An intimate role for adult dorsal root ganglia resident cycling cells in the generation of local macrophages and satellite glial cells. J Neuropathol Exp Neurol. 2018. 77: 929-41
9. Krispin S, Nitzan E, Kalcheim C. The dorsal neural tube: A dynamic setting for cell fate decisions. Dev Neurobiol. 2010. 70: 796-812
10. Kural C, Guresci S, Simsek GG, Arslan E, Tehli O, Solmaz I. Histological structure of filum terminale in human fetuses. J Neurosurg Pediatr. 2014. 13: 362-7
11. Lendon RG, Emery JL. Heterotopic dorsal-root ganglion cells around the spinal cord in children with spina bifida aperta. Dev Med Child Neurol Suppl. 1976. 37: 16-21
12. Morioka T, Hashiguchi K, Yoshida F, Nagata S, Miyagi Y, Mihara F. Dynamic morphological changes in lumbosacral lipoma during the first months of life revealed by constructive interference in steady-state (CISS) MR imaging. Childs Nerv Syst. 2007. 23: 415-20
13. Morioka T, Suzuki SO, Murakami N, Shimogawa T, Mukae N, Inoha S. Neurosurgical pathology of limited dorsal myeloschisis. Childs Nerv Syst. 2018. 34: 293-303
14. Morioka T, Suzuki SO, Murakami N, Mukae N, Shimogawa T, Haruyama H. Surgical histopathology of limited dorsal myeloschisis with flat skin lesion. Childs Nerv Syst. 2019. 35: 119-28
15. Morioka T, Murakami N, Kurogi A, Mukae N, Shimogawa T, Shono T. Embryopathological relationship between retained medullary cord and caudal spinal lipoma. Interdiscip Neurosurg. 2022. 29: 101534
16. Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017. 19: 428-39
17. Mukae N, Morioka T, Suzuki SO, Murakami N, Shimogawa T, Kanata A. Two cases of large filar cyst associated with terminal lipoma: Relationship with retained medullary cord. World Neurosurg. 2020. 142: 294-8
18. Murakami N, Morioka T, Shimogawa T, Mukae N, Inoha S, Sasaguri T. Ependyma-lined canal with surrounding neuroglial tissues in lumboscaral lipomatous malformations: Relationship with retained medullary cord. Pediat Neurosurg. 2018. 53: 387-94
19. Reinhold AK, Rittner HL. Characteristics of the nerve barrier and the blood dorsal root ganglion barrier in health and disease. Exp Neurol. 2020. 327: 113244
20. Ross GW, Swanson SA, Perentes E, Urich H. Ectopic midline spinal ganglion in diastematomyelia: A study of its connections. J Neurol Neurosurg Psychiatry. 1988. 51: 1231-4
21. Shirozu N, Morioka T, Inoha S, Imamoto N, Sasaguri T. Enlargement of sacral subcutaneous meningocele associated with retained medullary cord. Childs Nerv Syst. 2018. 34: 1785-90
22. Tosney KW. The early migration of neural crest cells in the trunk region of the avian embryo: An electron microscopic study. Dev Biol. 1978. 62: 317-33
23. Yang J, Lee JY, Kim KH, Wang KC. Disorders of secondary neurulation: Mainly focused on pathoembryogenesis. J Korean Neurosurg Soc. 2021. 64: 386-405