- Department of Neurosurgery, American Hospital, Istanbul, Turkey, Turkey
- Department of Neurosurgery, Istanbul Physical Therapy and Rehabilitation Training Hospital, Istanbul, Turkey
- Department of Neurology, School of Medicine, Boston University, Boston, MA, USA
- Department of Pathology, School of Medicine, Acibadem University, Istanbul, Turkey
- Department of Biochemistry, Faculty of Medicine, Marmara University, Istanbul, Turkey
- Department of Neurosurgery, School of Medicine, Koc University, Istanbul, Turkey
Correspondence Address:
Mehdi Sasani
Department of Neurosurgery, School of Medicine, Koc University, Istanbul, Turkey
DOI:10.4103/2152-7806.180092
Copyright: © Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sasani M, Ahmet Levent Aydın, Aytan N, Yapicier O, Oktenoglu T, Ozer NK, Ozer AF. Effect of a hypercholesterolemia as a starting factor on spinal degeneration in rabbits and role of Vitamin E (α-tocopherol). Surg Neurol Int 11-Apr-2016;7:36
How to cite this URL: Sasani M, Ahmet Levent Aydın, Aytan N, Yapicier O, Oktenoglu T, Ozer NK, Ozer AF. Effect of a hypercholesterolemia as a starting factor on spinal degeneration in rabbits and role of Vitamin E (α-tocopherol). Surg Neurol Int 11-Apr-2016;7:36. Available from: http://surgicalneurologyint.com/surgicalint_articles/effect-of-a-hypercholesterolemia-as-a-starting-factor-on-spinal-degeneration-in-rabbits-and-role-of-vitamin-e-%ce%b1%e2%80%91tocopherol/
Abstract
Background:To identify the role of the hypercholesterolemia as a starting factor in discovertebral degeneration that ultimately causes lower back pain, and investigate the role of Vitamin E in this process.
Methods:The rabbits (n = 32) were divided into two broad experimental groups: A control group, and a hypercholesterolemia group, namely cholesterol, and cholesterol plus Vitamin E groups and they were fed sequentially for 4 or 8 weeks. Serum cholesterol and Vitamin E (α-tocopherol) levels were determined; vascular tissue was prepared for histopathological analyses and vertebra was decalcified for the study.
Results:Cholesterol diet group resulted approximately 44-fold of increase plasma cholesterol levels over the 4-week control values. Additional supplementation with Vitamin E group induced a plasma cholesterol level increase of only 37-fold as compared to the control group. In the cholesterol groups, light microscope examination revealed atherosclerotic plaque in major arteries. However, in the cholesterol plus Vitamin E treatment groups, no lipid accumulation or foam cell formation was visible in the abdominal aorta and vertebral segmental artery. In histopathological examination, we found degenerative changes in the discovertebral unit in cholesterol treated groups.
Conclusion:Hypercholesterolemia causes fat accumulation in the disc endplate and vertebral body that causes blood supply disturbances which might be a starting factor of discovertebral degeneration. This event was not reversed by the elimination of cholesterol from the diet. Vitamin E supplementation was not effective in reducing fat accumulation in vertebral bone marrow. As a result, we conclude that degeneration of the discovertebral unit is not related to atherosclerotic changes in the major blood vessels.
Keywords: Atherosclerosis, disc degeneration, hypercholesterolemia, rabbit spine, spine degenerative disease, Vitamin E
INTRODUCTION
Low back pain resulting from lumbar degenerative disc disease is a common cause of morbidity and deterioration of the quality of life.[
It has been widely accepted that oxidative modification of low-density lipoprotein (LDL) cholesterol plays a pivotal role in the progression of atherosclerosis.[
In this study, we had two aims. First, we planned to investigate the general effects of hypercholesterolemia on the spine and endplates, specifically focusing on degeneration resulting from fatty replacement. Second, we examined if hypercholesterolemia related changes could be reversed by either Vitamin E or lack of cholesterol in the diet collectively. The study will help us to elucidate the relationship between hypercholesterolemia and atherosclerosis, as well as their mutual impact on spinal degeneration.
MATERIALS AND METHODS
Animal model
Male New Zealand albino rabbits, ranging in age from 2 to 4 months old, were randomly selected as the experimental groups. The animals were maintained and handled in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals prepared by the Animal Ethics Committee of Marmara University. All rabbits were fed 100g of rabbit food per day. Cholesterol was added to the diet as diethyl ether solution. The cholesterol concentration was chosen based on previously reported protocols.[
Experimental management
The rabbits (n = 32) were divided into two broad experimental groups: A control group and a hypercholesterolemia group. The control rabbits (n = 8) were fed only with Vitamin E-poor diet. The control group was divided into 1a and 1b groups. Four rabbits in Group 1a were fed for 4 weeks and the next four (Group 1b) for 8 weeks without any added supply and treatments. The hypercholesterolemia group rabbits (n = 24) were fed with the same Vitamin E-poor diet, except that it was supplemented with 2% cholesterol. This hypercholesterolemic group was divided into four subgroups (2a, 2b, 2c, and 2d). Rabbits in subgroup 2a (n = 6) were fed for 4 weeks. Members of Group 2b (n = 6) were fed with the same diet + 2% cholesterol but also received daily intramuscular injections of 50 mg/kg of Vitamin E for 4 weeks. The third (2c, n = 6) and fourth (2d, n = 6) subgroups were fed with a diet containing 2% cholesterol for first 4 weeks. Then, the rabbits in Group 2c received intramuscular Vitamin E (50 mg/kg) injections, while those in Group 2d were fed only with the Vitamin E-poor diet for an additional 4 weeks. After either 4 weeks (in Groups 1a, 2a, and 2b) or 8 weeks (in Groups 1b, 2c, and 2d) the rabbits were subjected to overnight food withdrawal and were subsequently anesthetized using 50 mg/kg of ketamine hydrochloride. Blood was collected to evaluate Vitamin E and cholesterol levels. The vertebral segmental arteries of all the rabbits were removed for microscopic evaluation. Then a posterior median skin incision was made on the lumbar paravertebral muscles, allowing dissection and complete removal of the lumbar columna vertebralis.
Determination of serum cholesterol and Vitamin E (α-tocopherol)
Serum cholesterol levels were determined using an automated (Hitachi Modular P800) enzymatic technique (Roche, Boehringer Inelheim).
Vitamin E levels were measured by reverse-phase high-pressure liquid chromatography (RP-HPLC).[
Preparation of vascular tissue for histopathological analyses
The vertebral segmentary arteries from all groups were fixed for histopathology in 10% buffered paraformaldehyde for a minimum of 24 h. Tissue samples were prepared in an autotechnicon and then embedded in paraffin. The specimens were sectioned (5 μm) with a microtome and deparaffinated 3 times with xylene in a 60°C incubator. The tissue samples were rehydrated with alcohol, washed with water, and stained with hematoxylin and eosin (H and E). The morphometric characteristics of tissue samples were evaluated under a light microscope (magnification, ×100 and × 400).
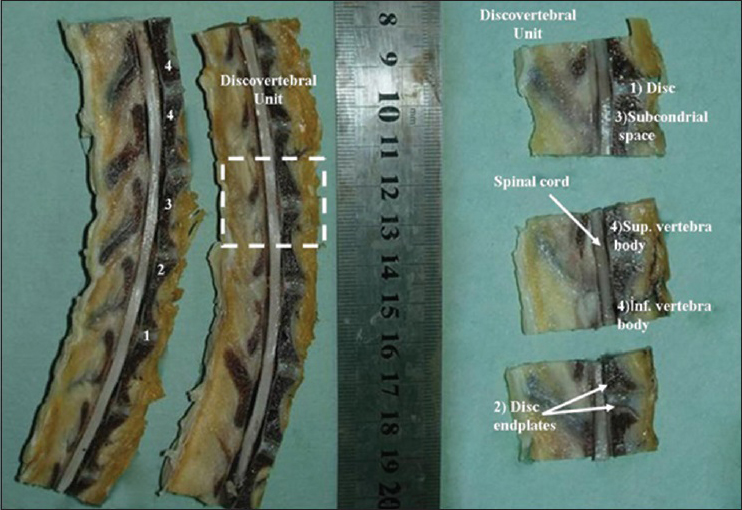
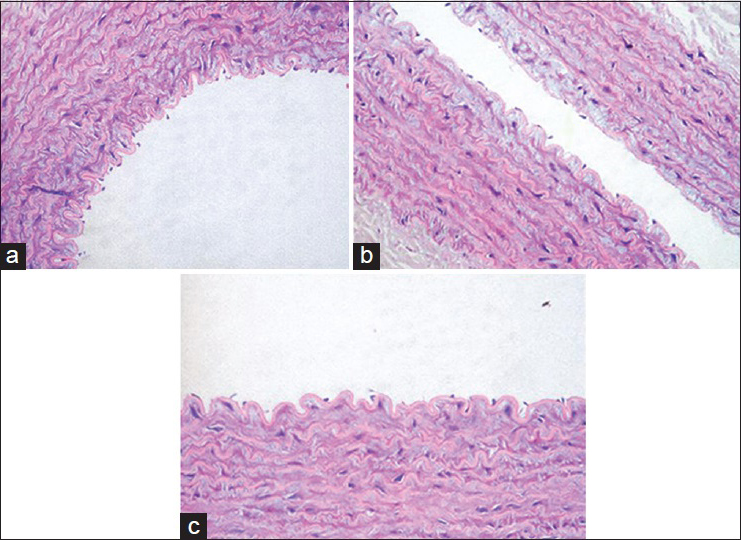
Decalcification and preparation of vertebrae
The specimens were fixed in a 10% buffered paraformaldehyde for 24 h, and then the osseous parts of the vertebrae were put in a decal solution (Shandon TBD-1, 14% hydrochloric acid) for another 24 h. The specimen was serially sectioned in sagittal and axial planes and embedded in paraffin. A guillotine was used for the serial sectioning [
Results and statistical analyses
Cholesterol and Vitamin E serum concentrations
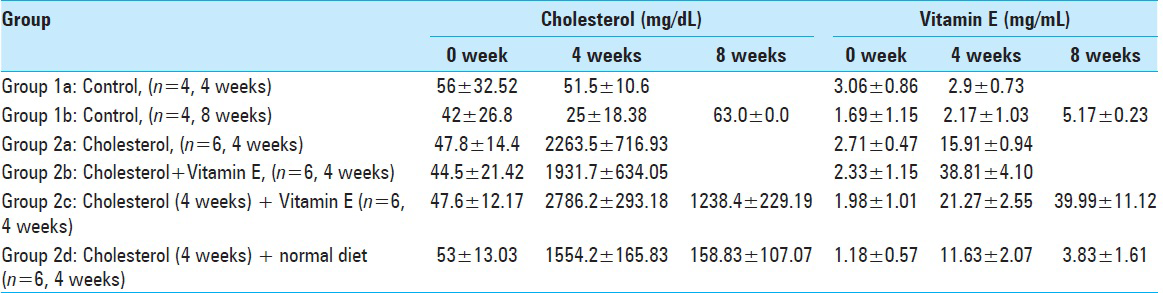
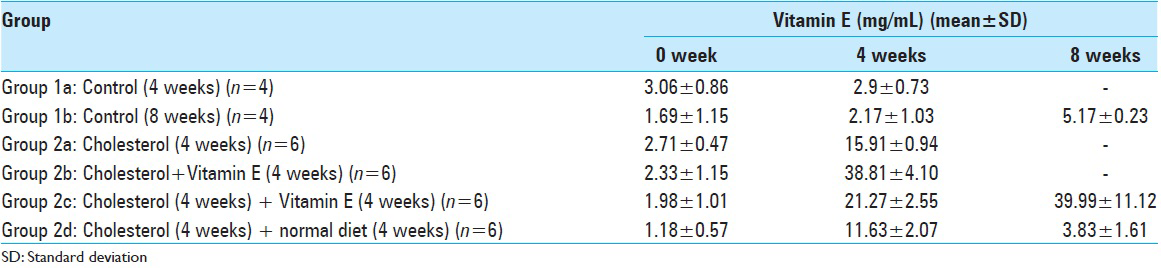
Blood analyses for the all experimental groups are shown in Tables
Statistical analyses
For the statistical analyses, Number Cruncher Statistical System 2007, Power Analysis and Sample Size 2008 Statistical Software Kaysville, Utah USA were used.
To evaluate the data of the study, descriptive statistical methods as a mean average and standard deviation were referred.
For the comparison of the results of qualitative data of two separate groups exhibiting nonuniform parameters, Mann–Whitney U-test was used.
To compare the qualitative data of nonuniform parameters in the same group, Wilcoxon signed ranks test was used.
Significance level was accepted to be at P < 0.01 and P < 0.05.
Interpretation of the statistical result
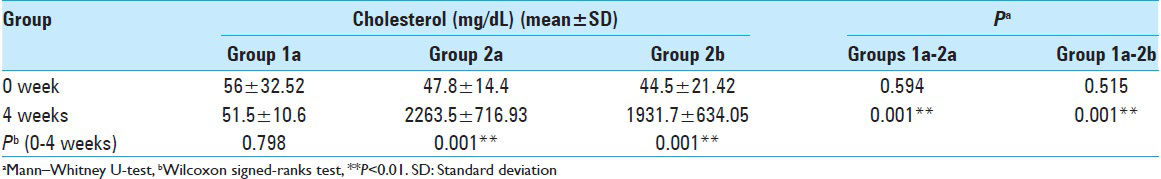
Evaluation of the results of different groups (1a vs. 2a and 1a vs. 2b) [Tables 1 – 3 ]
Considering the initial level of serum cholesterol, there was no significant statistical difference among Groups 1a (fed with normal diet for 4 weeks) and 2a (fed with cholesterol diet for 4 weeks) (P > 0.05). This means the two groups were uniform at the beginning, regarding the serum cholesterol levels.
As expected, the cholesterol levels of the Group 1a rabbits were lower than Group 2a, fed with high cholesterol diet at the end of 4 weeks; and this was statistically significant (P < 0.001; P < 0.01).
The initial cholesterol level of the Groups 1a (fed with a normal diet for 4 weeks) and 2b (fed with Vitamin E diet) was statistically insignificant (P > 0.05). This also means that these two groups were uniform at the beginning of the study, regarding the serum cholesterol levels.
The cholesterol level of the Group 1a rabbits was lower than the Group 2b rabbits at the end of 4 weeks and this was statistically significant (P: 0.001; P < 0.01), showing that the supplementation with Vitamin E did not preserve the serum cholesterol profile at the Group 2b at the end of the 4th week.
Evaluation of the results within the groups (1a, 2a, and 2b separately) [Tables 1 and 3 ]
Decrease of the serum cholesterol level of the Group 1a (fed with a normal diet for 4 weeks) rabbits at the end of the 4th week was found to be statistically insignificant (P > 0.05).
Increase of the serum cholesterol level of the Group 2a rabbits (fed with high cholesterol diet for 4 weeks) at the end of 4 weeks was found to be statistically significant (P: 0.001; P < 0.01) as expected.
Increase of the serum cholesterol level of the Group 2b rabbits (fed with high cholesterol and Vitamin E diet for 4 weeks) at the end of 4 weeks was found to be statistically significant (P: 0.001; P < 0.01).
Evaluation of the result of different groups (1b vs. 2c and 1b vs. 2d) [Tables 1 and 3 ]
There was no statistically significant difference among the initial cholesterol levels of the Group 1b rabbits (fed with a normal diet for 8 weeks) and Group 2c rabbits (fed with high cholesterol diet for the first 4 weeks, and with Vitamin E diet or the next 4 weeks) (P > 0.05).
The fourth-week cholesterol level of the Group 1b rabbits was statistically significantly lower than those of Group 2c (P: 0.001; P < 0.01).
The 8 weeks cholesterol level of the Group 1b rabbits was statistically significantly lower than the Group 2c rabbits (P: 0.001; P < 0.01).
The initial cholesterol levels of the Group 1b rabbits (fed with a normal diet for 8 weeks) and 2d rabbits (fed with cholesterol diet for 4 weeks and with a normal diet for the next 4 weeks) were statistically similar (P > 0.05).
The cholesterol level at the end of the 4th week of Group 1b was statistically significantly lower than Group 2d (P: 0.001; P < 0.01).
The cholesterol level at the end of 8 weeks of Group 1b was statistically significantly lower than Group 2d (P: 0.001; P < 0.01).
Evaluation of the results within the Groups 1b, 2c, and 2d separately [Tables 1 and 4 ]
Decrease of the cholesterol level of the Group 1b at the end of the 4th week, according to the initial level was found to be statistically insignificant (P > 0.05). Either, the increase of the cholesterol level of this group at the end of the 8 weeks, according to the initial level was found to be insignificant (P > 0.05).
Considering the Group 2c rabbits, fed with cholesterol diet for the initial 4 weeks, and with Vitamin E diet for the next 4 weeks, the increase of the cholesterol level at the end of the 4th week, according to the initial level was found to be statistically significant (P: 0.001; P < 0.01).
Besides, the increase of the cholesterol level of this group at the end of the 8 weeks, according to the initial level was found to be statistically significant (P: 0.001; P < 0.01).
The increase of the 4th week cholesterol level according to the initial level of the Group 2d rabbits, fed with cholesterol diet at the first 4 weeks, and with normal diet at the next 4 weeks was found to be statistically significant (P: 0.001; P < 0.01).
The increase in the cholesterol level of this group at the end of 8 weeks was found to be statistically significant (P: 0.012; P < 0.05).
RESULTS
Histopathological analyses of vascular tissue
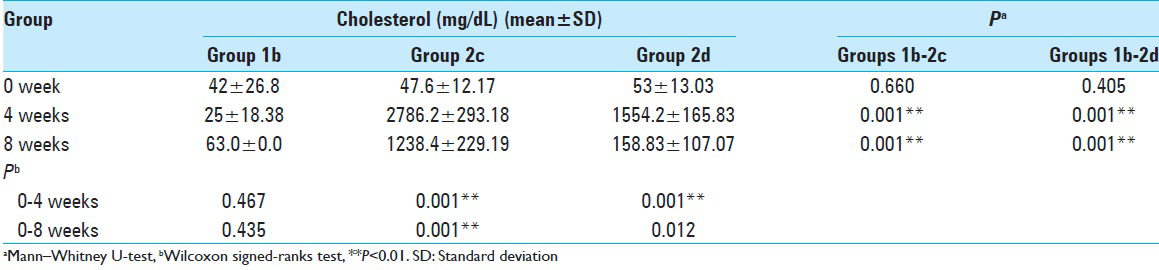
Rabbit abdominal aortas and vertebral segmental arteries from the different experimental groups were studied. Representative light microscopy images of the vertebral segmental artery from control rabbits [
Figure 2
Representative light microscopy images of the rabbit segmental artery from: (a) control rabbits, (b) rabbits from the cholesterol group, which showed atherosclerotic vascular with foam cells in the subendothelial and medial layers and endothelial desquamation (arrows), (c) rabbits from the cholesterol plus Vitamin E treatment group, which showed no lipid accumulation or foam cell formation (×400)
Histopathological analyses of discs, disc endplates, and vertebras
In the control group, normal histological findings in the annulus and endplate, along with normal neural elements of the spinal cord and nerve roots were observed. A discovertebral unit is comprised of vertebral bone marrow, disc endplates, and the disc component. In rabbits from the control groups, the endplate was composed of an osseous component as well as hyaline cartilage material. The disc endplate is considered to be the thin layer of hyaline cartilage that lies between the bone of the vertebral body and the soft tissue of the disc. In rabbits, the disc endplates are typically <0.1 mm [
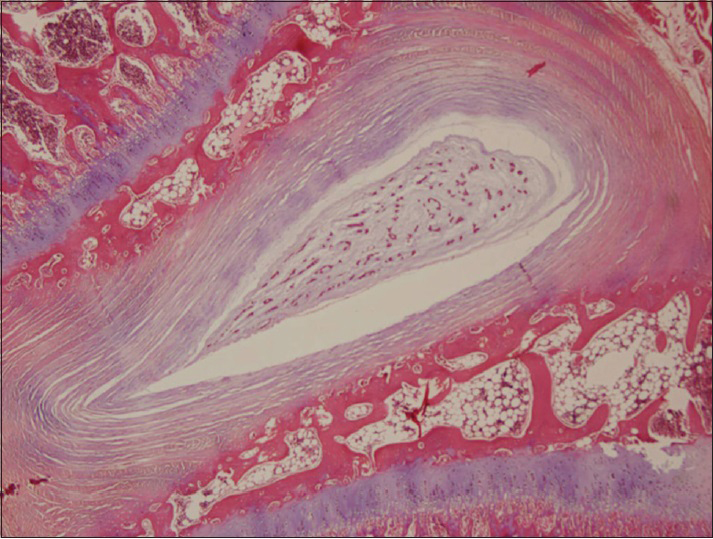
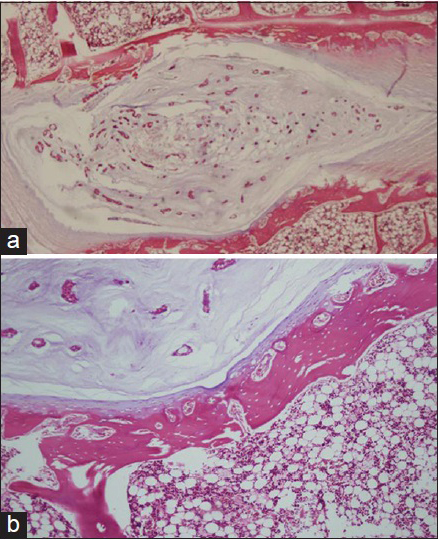
In our histopathological examination, we found degenerative changes in the discs, disc endplates, and vertebral bodies of the cholesterol treated rabbits for 4 weeks. Accumulation of foam cells (lipid deposits) in the disc endplates and bone marrow caused disruption of the blood supply. This process could mark the beginning of degenerative changes in disc endplates. In addition to this finding, there were solitary or aggregated foamy histiocytes (xanthic cells) in the cartilage, bone marrow, tendons, and soft tissue [
Figure 4
Histopathological examination; (a) 2% cholesterol feed group (×100) and (b) cholesterol plus Vitamin E treatment group demonstrated degenerative changes in the discs, disc endplates and vertebral bodies, accumulation of foamy histiocytes (xanthic cells) in the disc endplates and bone marrow caused disruption of the blood supply (black arrows) (×400)
In rich cholesterol-fed plus Vitamin E-supplement groups for 4 weeks, histopathological examination revealed changes in the discovertebral unit that were similar to those in the rich cholesterol-fed groups. We did not find any discernible decreases in the number of foam cells related to Vitamin E, whereas Vitamin E certainly had a significant effect on atherosclerotic lesions in the large arteries of these same rabbits. Therefore, we concluded that Vitamin E was insufficient to diminish foam cells, despite its effect on atherosclerotic lesions in large vessels for this treatment period [
In rich cholesterol-fed plus Vitamin E-supplemented groups for 8 weeks (first 4 weeks rich cholesterol + second 4 weeks Vitamin E supplement), histopathological examination did not reveal discernible decreases in the number of foam cells in spite of the supplementation of Vitamin E on second 4 weeks. In rich cholesterol-fed and normal diet groups for 8 weeks ( first 4 weeks rich cholesterol + second 4 weeks normal diet), we observed no changes in discovertebral fat accumulation on second 4 week. We conclude that hypercholesterolemia causes degenerative changes in the discovertebral unit [
DISCUSSION
Lumbar arterial supply may be diminished in certain situations, and this causes intervertebral disc degeneration and low back pain. The diminished vertebral blood supply is thought to be a result of atherosclerosis and/or stenosis of lumbar arteries. Lumbar vascular supply is segmental. Lumbar ischemia may have several different outcomes, affecting structures such as nerve roots, vertebral bodies, intervertebral discs, and paravertebral muscles. Depending on the structures nourished by blood, symptoms may vary: Ischemia of the vertebral bone may cause continuous dull pain, typical of skeletal ischemia. Nerve root ischemia may evoke radicular pain. The intervertebral disc is supplied by diffusion through the endplates from vertebral bodies above and below it. Furthermore, the disc is located at the end of oxygen and nutrient chain, making it the first structure to suffer during nutrient insufficiency.[
Hypercholesterolemia plays an important role in the progression of atherosclerosis.[
As the pathophysiological mechanisms of inflammation are similar in vascular tissue (atherosclerosis) and cartilage tissue, we assume that hypercholesterolemia may contribute to intervertebral disc degeneration by the same mechanism of inflammation and by affecting the vessels supplying the disc tissue. The damaged synovial membrane, calcified cartilage, neighboring bone tissue, and angiogenesis in turn may lead to the vertebral body Modic degeneration and at the same time, causing prominent atherosclerosis in the aorta and vessels nourishing the vertebral column.
The protective effects of antioksidants such as αT (Vitamin E) on bone metabolism have been studied in animals and humans, and these studies show generally the beneficial effects of this metabolite on bone metabolism. There are a number of studies in the literature about the relationship between hypercholesterolemia, αT (Vitamin E) supplementation and bone and cartilage metabolism, and vascular changes. These studies try to focus on the alterations of the histologic structure of vascular, osseous, and cartilage tissue caused by cholesterol increase and whether these alterations can be reversed by supplementing Vitamin E.
Kasai et al. studied the effect of αT on the vertebral and femur bone of ovariectomized rats. They found that αT supplementation caused a tendency for the bone mass increase and remodeling of the vertebral body in normal rats.[
Hampson et al. studied αT relationship with bone metabolism and arterial stiffness in postmenopausal women and they concluded that high serum concentrations of αT might have a negative effect on bone formation.[
The effect of hypercholesterolemia on synovial and vascular endothelial structure has been discussed in the literature. Similar to our study, Prieto-Potín et al. induced hypercholesterolemia in rabbits by administrating high-fat diet and analyzed lipid and systemic markers in blood serum. We can assume that vertebral unit is a kind of joint structure, composed of bone (vertebral body), cartilage (end plate and disc tissue), and synovium (lining facet cartilage), and the results of Potin's study could be simulated to our study, as they examined knee joint.
Hypercholesterolemia causes cartilage-bone damage, and foam macrophages are the main causes in this process. Inflammatory actions induced by adipokines explain some of the associations between skeletal and cardiovascular effects of hypercholesterolemic cases. The biochemical causes of disc-cartilage inflammation and their vascular adverse effects may be related to cytokines, chemokines, adipokines such as tumor necrosis factor-alpha, interleukin-6, plasminogen activator inhibitor, end others. Endothelial dysfunction is a result of these chemokines and inflammatory molecules, causing increased vessel permeability. Leukocytes rush toward the synovia, cartilage. Lipid infiltration, foam cell, and fatty streaks within the vessel wall, eventually cause atherosclerotic plaques. Mainly, macrophage infiltration promotes atherosclerosis and also causes cartilage changes. Macrophages act as antigen presenting cells and finally cause inflammation in acute and chronic cases.
Besides, macrophages may be transformed to cathepsin K-stained osteoclasts and cathepsin K is the major osteoclast protease responsible for bone resorption. Thus, this metabolic event in hyperlipidemia causes bone degeneration. A terminal degradation product of these osteoclasts, C-terminal telopeptide of Type I collagen may be used as an indicator of bone resorption, degeneration and also it is found in atherosclerotic plaques.[
The mononuclear cells infiltrating to the vascular wall and cartilage differentiate to osteoclast-like cells and cause bone resorption. This aggressive infiltration eventually causes degenerative changes in the joints, vertebral bone, and vascular tissue.
Bone quality parameters are altered by a hypercholesterolemic diet in animal experimental studies, including bone volume fraction, bone mineral density, trabecular number, alterations in mechanical properties of the bone, and an increase in osteoclast number.[
Hyperlipidemia increases systemic inflammatory effects on the vascular bone and cartilage tissues by causing massive macrophage infiltration and transformation to foam cells and osteoclasts and in turn leading to atherosclerosis and disc and vertebral degeneration.
The role of αT (Vitamin E) has been studied in hypercholesterolemia as an antioxidant, primarily for preventing the vascular effects. Vitamin E supplementation may be associated with a lower risk of atherosclerosis and lower risk of coronary heart disease, but it is dose dependent. High-dose Vitamin E supplementation (over 400 IU/day) may be associated with an increased mortality rate. Besides the vascular effects, osteogenic effects of Vitamin E have been studied widely. Vitamin E is shown to cause bone mass increase in cancellous bones of the vertebral body, leading to beneficial effects on bone health. The osteoclast activation of free radicals, affecting bone turnover is reversed in the benefit of bone remodeling by antioxidants as Vitamin E. The underlying mechanism has not been determined thoroughly yet.[
In this study, we evaluated the effect of a hypercholesterolemia as a starting factor on spinal degeneration in an experimental animal model (rabbits) and the role of Vitamin E. A high cholesterol supplementation was preferred to lower cholesterol-containing diets (5–10 g/kg). This decision turned out to have the advantage of maximizing the atherosclerotic result and diminishing the treatment time as a result elevation in blood cholesterol in rabbits far exceed that seen in the most humans. Progressive degeneration of the disc endplates and changes to the vertebral body were found to be correlated with high blood concentrations of cholesterol. The noticeable concession of the current study, when compared to similar studies is that not only the effect of hypercholesterolemia and Vitamin E on the major vascular tissue and on the discovertebral unit was investigated, but also serum concentration, vascular and discovertebral unit histological changes were examined at the same study. One limitation of the study was that it encompassed a relatively short period of investigation (4 and 8 weeks). However, the length of the time period was proved to be sufficient for revealing vascular changes, such as plaque formation, which is associated with hypercholesterolemia and which was congruent with the time frame of the previous publications in the literature.[
Disc degeneration is a very complex phenomenon that will require extensive biomechanical and biochemical researches before it will be fully understood.[
Atherosclerosis of aorta and stenosis of the lumbar spine feeding arteries are all proven to be associated with degenerative disc disease. Cardiovascular risk factors of atherosclerosis on the other hand are also proven by many studies.
The discovertebral junction is a dynamic zone that demonstrates normal variations and can be modified by disease, which makes biomechanical and biochemical investigations inevitable in the study of degeneration. Possible support for the association between endplate degeneration and changes in subchondral bone and bone marrow include histological specimens from groups that were given food containing 2% cholesterol. As a consequence of high cholesterol diet increased oxidative stress and vascular smooth muscle cell proliferation may initiate, release of inflammatory parameters and other locally liberated factors and has been assumed to be important in the development of vascular and discovertebral unit histological changes.[
Vitamin E (αT) has been shown to regulate a number of cellular processes, including signal transduction and cell proliferation.[
A high plasma concentration of cholesterol, in particular high levels of LDL, is one of the principal risk factors for atherosclerosis. The lesions occur in muscular arteries and can lead to ischemia of the organs that are supplied by the atherosclerotic artery. Necropsy studies have demonstrated a marked association between atheromatous lesions in the aorta and lumbar disc degeneration and stenosis of the feeding arteries of the lumbar vertebrae. These changes in turn lead to lumbar back pain.[
Besides, the lack of effectiveness of the Vitamin E in preventing the discovertebral degeneration may be due to the duration of the experiment (8 weeks). Vitamin E might be effective in preventing the discovertebral degeneration in a longer time period. On the other hand, vascularization of vertebral body is weak, particularly disc tissue has no vessels, but aorta is rich in vasa vasorum. According to our opinion, consequent researches are needed about this issue.
CONCLUSION
As a result, we conclude that degeneration of the discovertebral unit is not related to atherosclerotic changes in the large blood vessels, such as the aorta or spine segment artery.
Further experimental and prospective clinical studies are needed to clarify the association of atherosclerosis and low back disorders, disc and vertebral degeneration and the precautions to reverse this pathology.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
1. Bocan TM, Mueller SB, Mazur MJ, Uhlendorf PD, Brown EQ, Kieft KA. The relationship between the degree of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis. 1993. 102: 9-22
2. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976). 2002. 27: 2631-44
3. Cosar M, Iplikcioglu AC, Aytan N, Ozcan D, San T, Kartal-Ozer N. The effect of temporary aneurysm clip on the common carotid artery of atherosclerotic rabbits. Surg Neurol. 2008. 69: 483-8
4. Crowther MA. Pathogenesis of atherosclerosis. Hematology Am Soc Hematol Educ Program. 2005. 1: 436-41
5. Hampson G, Edwards S, Sankaralingam A, Harrington DJ, Voong K, Fogelman I. Circulating concentrations of Vitamin E isomers: Association with bone turnover and arterial stiffness in post-menopausal women. Bone. 2015. 81: 407-12
6. Hsieh AH, Hwang D, Ryan DA, Freeman AK, Kim H. Degenerative anular changes induced by puncture are associated with insufficiency of disc biomechanical function. Spine (Phila Pa 1976). 2009. 34: 998-1005
7. Karppinen J, Daavittila I, Solovieva S, Kuisma M, Taimela S, Natri A. Genetic factors are associated with modic changes in endplates of lumbar vertebral bodies. Spine (Phila Pa 1976). 2008. 33: 1236-41
8. Kasai S, Ito A, Shindo K, Toyoshi T, Bando M. High-dose α tocopherol supplementation does not induce bone loss in normal rats. PLoS One. 2015. 10: e0132059-
9. Kauppila LI, Tallroth K. Postmortem angiographic findings for arteries supplying the lumbar spine: Their relationship to low-back symptoms. J Spinal Disord. 1993. 6: 124-9
10. Kauppila LI. Atherosclerosis and disc degeneration/low-back pain – A systematic review. Eur J Vasc Endovasc Surg. 2009. 37: 661-70
11. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988. 166: 193-9
12. Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C. Modic changes and their associations with clinical findings. Eur Spine J. 2006. 15: 1312-9
13. Morel DW, de la Llera-Moya M, Friday KE. Treatment of cholesterol-fed rabbits with dietary Vitamins E and C inhibits lipoprotein oxidation but not development of atherosclerosis. J Nutr. 1994. 124: 2123-30
14. Natarajan RN, Williams JR, Andersson GB. Recent advances in analytical modeling of lumbar disc degeneration. Spine (Phila Pa 1976). 2004. 29: 2733-41
15. Negis Y, Aytan N, Ozer N, Ogru E, Libinaki R, Gianello R. The effect of tocopheryl phosphates on atherosclerosis progression in rabbits fed with a high cholesterol diet. Arch Biochem Biophys. 2006. 450: 63-6
16. Okayasu M, Nakayachi M, Hayashida C, Ito J, Kaneda T, Masuhara M. Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J Biol Chem. 2012. 287: 19229-41
17. Ozer NK, Azzi A. Effect of Vitamin E on the development of atherosclerosis. Toxicology. 2000. 148: 179-85
18. Ozer NK, Negis Y, Aytan N, Villacorta L, Ricciarelli R, Zingg JM. Vitamin E inhibits CD36 scavenger receptor expression in hypercholesterolemic rabbits. Atherosclerosis. 2006. 184: 15-20
19. Ozer NK, Sirikçi O, Taha S, San T, Moser U, Azzi A. Effect of Vitamin E and probucol on dietary cholesterol-induced atherosclerosis in rabbits. Free Radic Biol Med. 1998. 24: 226-33
20. Pelton K, Kreider J, Joiner D. Hypercholesterolemia promotes an osteoporotic phenotype. Am J Pathol. 2015. 15: 1-9
21. Prieto-Potín I, Roman-Blas JA, Martínez-Calatrava MJ, Gómez R, Largo R, Herrero-Beaumont G. Hypercholesterolemia boosts joint destruction in chronic arthritis. An experimental model aggravated by foam macrophage infiltration. Arthritis Res Ther. 2013. 15: R81-
22. Solgaard Sorensen J, Kjaer P, Jensen ST, Andersen P. Low-field magnetic resonance imaging of the lumbar spine: Reliability of qualitative evaluation of disc and muscle parameters. Acta Radiol. 2006. 47: 947-53
23. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989. 320: 915-24
24. Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004. 24: e6-10
25. Upston JM, Kritharides L, Stocker R. The role of Vitamin E in atherosclerosis. Prog Lipid Res. 2003. 42: 405-22
26. Waddell G. Low back pain: A twentieth century health care enigma. Spine (Phila Pa 1976). 1996. 21: 2820-5
27. Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011. 17: 1410-22
28. Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007. 6: 247-61
29. Zingg JM, Azzi A. Non-antioxidant activities of Vitamin E. Curr Med Chem. 2004. 11: 1113-33