Effect of cranioplasty timing on the functional neurological outcome and postoperative complications
- College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia,
- King Abdullah International Medical Research Center, Riyadh, Saudi Arabia,
- Division of Neurosurgery, Department of Surgery, Ministry of National Guard - Health Affairs, Riyadh, Saudi Arabia,
- University of Miami, Department of Neurological Surgery, Miami, FL. USA,
- Department of Neurosurgery, Prince Sultan Military Medical City, Riyadh, Arabia,
- Prince Mohammed Medical City, Jouf, Saudi Arabia,
- Faculty of Applied Medical Sciences, Jazan University, Jazan, Saudi Arabia,
- National Center for Evidence Based Healthcare, Saudi Health Council, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_802_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Aloraidi1,2,3, Ali Alkhaibary1,2,3, Ahoud Alharbi1,2,3, Nada Alnefaie1,2, Abeer Alaglan1,2,3, Abdulaziz AlQarni1,2,3, Turki Elarjani4, Ala Arab1,2,3, Jamal M. Abdullah5, Abdulaziz Oqalaa Almubarak6, Munzir Abbas1,2,3, Ibtesam Khairy7, Wedad H. Almadani8, Mohammed Alowhaibi2,3, Abdulaziz Alarifi2,3, Sami Khairy1,2,3, Ahmed Alkhani2,3. Effect of cranioplasty timing on the functional neurological outcome and postoperative complications. 07-Jun-2021;12:264
How to cite this URL: Ahmed Aloraidi1,2,3, Ali Alkhaibary1,2,3, Ahoud Alharbi1,2,3, Nada Alnefaie1,2, Abeer Alaglan1,2,3, Abdulaziz AlQarni1,2,3, Turki Elarjani4, Ala Arab1,2,3, Jamal M. Abdullah5, Abdulaziz Oqalaa Almubarak6, Munzir Abbas1,2,3, Ibtesam Khairy7, Wedad H. Almadani8, Mohammed Alowhaibi2,3, Abdulaziz Alarifi2,3, Sami Khairy1,2,3, Ahmed Alkhani2,3. Effect of cranioplasty timing on the functional neurological outcome and postoperative complications. 07-Jun-2021;12:264. Available from: https://surgicalneurologyint.com/surgicalint-articles/10863/
Abstract
Background: The optimal timing for performing cranioplasty and its effect on functional outcome remains debatable. Multiple confounding factors may come into role; including the material used, surgical technique, cognitive assessment tools, and the overall complications. The aim of this study is to assess the neurological outcome and postoperative complications in patients who underwent early versus late cranioplasty.
Methods: A retrospective cohort study was conducted to investigate the neurological outcome and postoperative complications in patients who underwent cranioplasty between 2005 and 2018 at a Level l trauma center. Early and late cranioplasties were defined as surgeries performed within and more than 90 days of decompressive craniectomy, respectively. The Glasgow Outcome Score (GOS) and modified Rankin scale (mRS), recorded within 1 week of cranioplasty, were used to assess the neurological outcome.
Results: A total of 101 cases of cranioplasty were included in the study. The mean age of the patients was 31.4 ± 13.9 years. Most patients (n = 86; 85.1%) were male. The mean GOS for all patients was 4.0 ± 1.0. The mean mRS was 2.2 ± 1.78. Hydrocephalus was noted in 18 patients (early, n = 6; late, n = 12; P = 0.48). Seizures developed in 28 patients (early, n = 12; late, n = 16; P = 0.77).
Conclusion: The neurological outcome in patients who underwent early versus late cranioplasty is almost identical. The differences in the rates of overall postoperative complications between early versus late cranioplasty were statistically insignificant. The optimal timing for performing cranioplasty is mainly dependent on the resolution of cerebral swelling.
Keywords: Bone flap, Comparison, Glasgow Outcome Scale, Modified Rankin scale
INTRODUCTION
Although cranioplasty is a common neurosurgical procedure, it carries a high complication rate. The complication rates of cranioplasty may reach up to 34%.[
Considering the high complication rates of cranioplasty, it is necessary to compare the neurological outcome relative to the timing of cranioplasty after decompressive craniectomy (DC). The purpose of the present study is to assess the neurological outcome and postoperative complication rates among patients who underwent early versus late cranioplasty at a major Level I trauma center.
MATERIALS AND METHODS
Patients’ eligibility and study setting
This is a retrospective cohort study, reviewing the neurological outcome and postoperative complications, in cranioplasty patients. Data were collected for all eligible cranioplasties performed between January 2005 and December 2018 at King Abdulaziz Medical City, Riyadh, Saudi Arabia. King Abdulaziz Medical City is a Level l trauma center serving Riyadh region and receives referrals from all over the country. King Abdulaziz Medical City was one of the main contributors to DC in diffuse traumatic brain injury (TBI) (DECRA Trial).[
All patients who underwent cranioplasty, preserved in the abdominal pocket, during the specified period were included in the study. The exclusion criteria were; patients with congenital cranial defects repaired by cranioplasty, patients who had nonautologous cranioplasty, and patients who had more than 50% of the defect replaced with nonautologous bone even in the presence of his/her bone. In addition, all patients who did not have documented primary outcome postoperatively or on follow-up visits were excluded from the study.
Data collection
Data were retrieved from the archives of the neurosurgery department using two distinct methods; medical record files from 2005 to 2015 and the hospital’s electronic system from 2016 to 2018. Data included patients’ demographics (age, gender, and body mass index [BMI]) and postoperative complications (hydrocephalus, hygroma, seizure, sunken flap syndrome, and long-term mortality). The primary indications for performing DC were TBI and malignant cerebral infarction. Comorbidities refer to the confirmed diagnosis of diabetes, hypertension, or cancer.
Early and late cranioplasties were defined as surgeries performed within and more than 90 days of DC, respectively. Long-term mortality was defined as death after 6 months of cranioplasty. [
The Glasgow Outcome Score (GOS) and modified Rankin scale (mRS) were calculated to assess the neurological outcome. The GOS and mRS were recorded within 1 week of performing cranioplasty. The GOS is scored as follows; (1 = Death), (2 = Persistent vegetative state), (3 = Severe disability), (4 = Moderate disability), and (5 = Good recovery).[
Statistical analysis
Data were coded and entered into IBM SPSS (version 23, IBM Corporation, Armonk, New York, United States). Descriptive statistics were performed to present categorical data as frequencies and percentages. Mean and standard deviation were calculated for numerical variables including; age, GOS, mRS, BMI, and intensive care unit stay. The independent sample T-test was performed to calculate the mean differences in numerical data between early versus late cranioplasty. The Chi-square test was performed to assess the association between categorical data and the timing of cranioplasty. The statistical significance level of the P-value was set at 0.05.
Ethical considerations
Ethical approval was obtained from the Institutional Review Board at King Abdullah International Medical Research Center (KAIMRC), Ministry of National Guard - Heath Affairs (NGHA), Riyadh, Saudi Arabia. Patients’ identities were kept concealed and deidentified. The study was noninterventional and retrospective in nature.
RESULTS
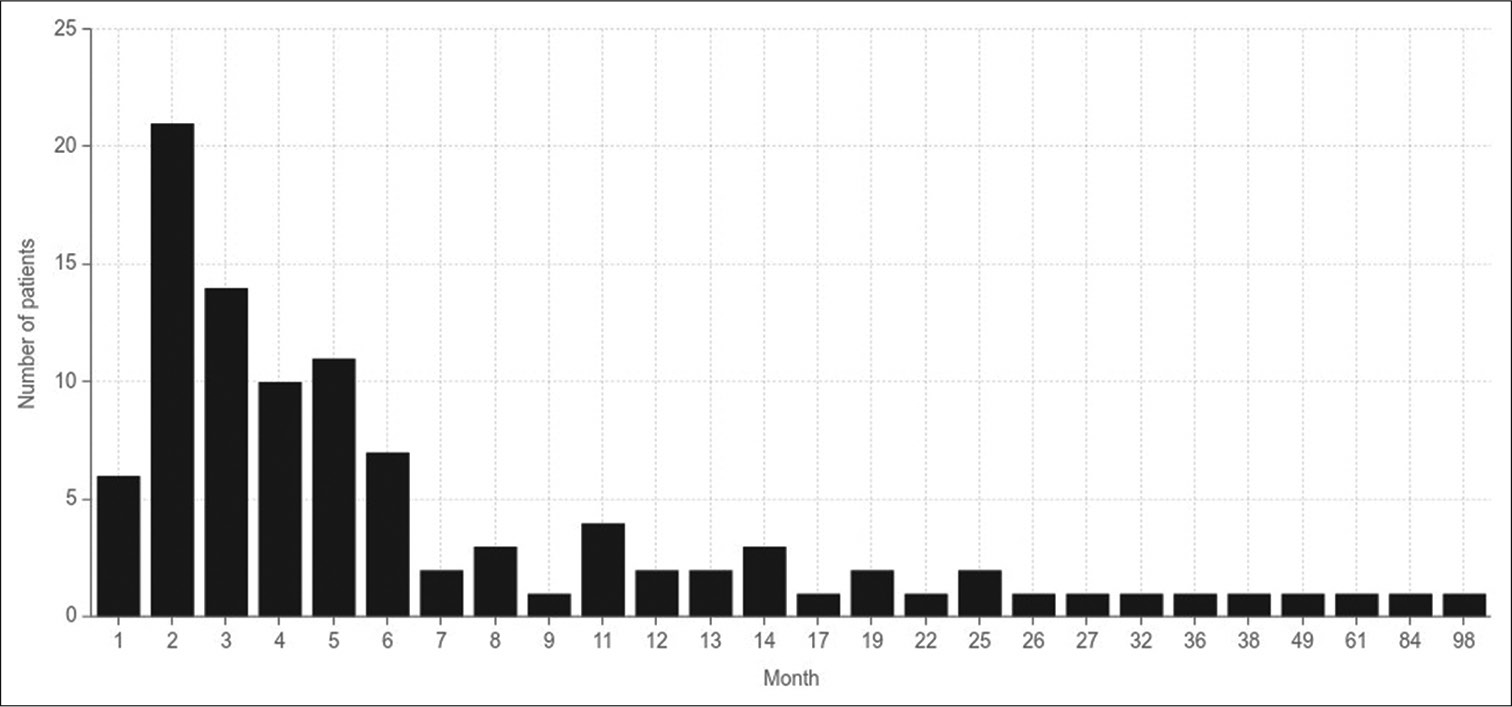
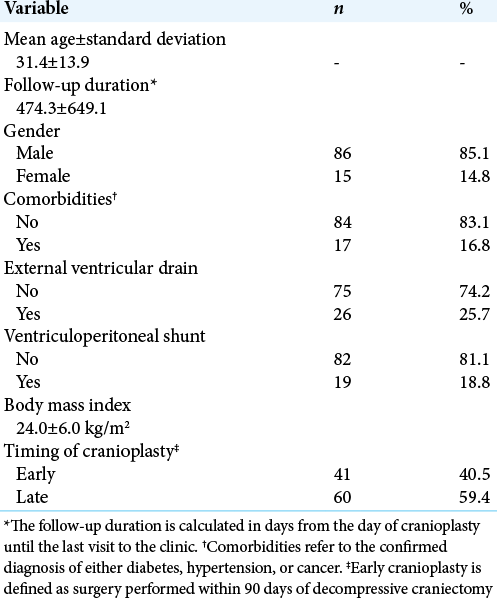
A total of 101 cases of autologous cranioplasty were included in the study. The mean age of the patients was 31.4 ± 13.9 years. The duration of follow-up for all patients following cranioplasty was 474.3 ± 649.1 days. The mean GOS for all patients was 4 ± 1. The mean mRS was 2.2 ± 1.78. Most patients (n = 86; 85.1%) were male. The mean BMI was 24.0 ± 6.0 kg/. Most cranioplasty cases were performed late (n = 60; 59.4%). Approximately one-quarter of the patients (n = 26; 25.7%) required external ventricular drainage (EVD) insertion. A total of 19 patients (18.8%) were committed to shunt dependency surgery, that is, ventriculoperitoneal shunt. [
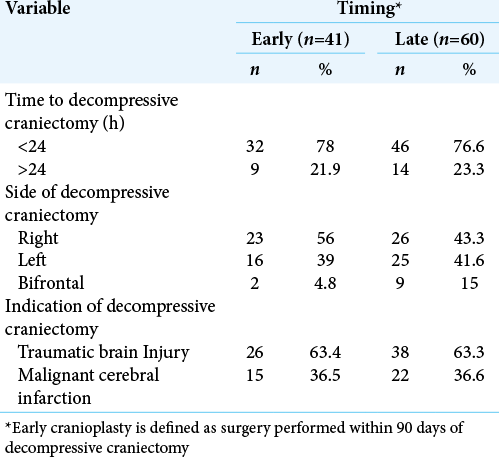
Among the cohort who underwent early cranioplasty, most decompressive craniectomies (n = 32; 78%) were performed within 24 h of admission. A total of 23 (56%) cases of decompressive craniectomies were right sided in the early cranioplasty group. TBI was the indication to perform DC in around two-thirds (n = 26; 63.4%) of the patients. In contrast, in the late cranioplasty cohort, a total of 14 (23.3%) patients underwent DC after 24 h of presentation to the emergency department. A total of 9 (15%) patients underwent bifrontal DC. The most frequent indication of DC was TBI (n = 38; 63.3%). [
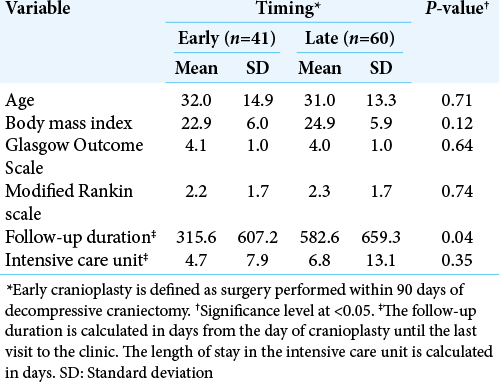
The mean age of patients who underwent early versus late cranioplasty was 32.0 ± 14.9 and 31 ± 13.3 years, respectively (P = 0.71). The mean BMI in early cranioplasty was 22.9 kg/m2 as opposed to 24.9 kg/m2 in late cranioplasty. The mean GOS for patients who underwent early and late cranioplasty was 4.1 ± 1.0 and 4.0 ± 1.0, respectively (P = 0.64). The mean mRS for patients with early cranioplasty was 2.2 ± 1.7. Patients who underwent late cranioplasty had an average mRS of 2.3 ± 1.7. [
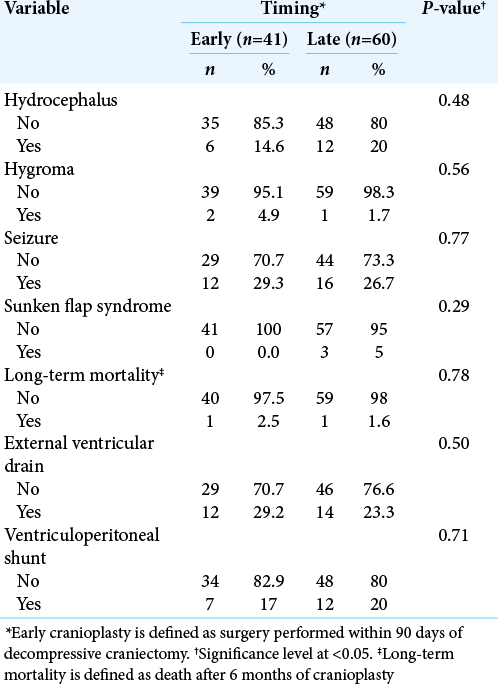
Hydrocephalus was identified in a total of 18 patients (early, n = 6; late, n = 12; P = 0.48). Hygroma developed in three patients (early, n = 2; late, n = 1; P = 0.56). A total of 28 patients presented with seizures postoperatively (Early, n = 12; Late, n = 16; P = 0.77). Sunken flap syndrome was noted in three patients (early, n = 0; late, n = 3; P = 0.29). Long-term mortality was encountered in two patients (early, n = 1; late, n = 1; P = 0.78). [
DISCUSSION
The present study investigated the association of the neurological outcome and postoperative complications relative to the timing of cranioplasty at a Level l trauma center. The overall postoperative complication rates between patients who underwent early versus late cranioplasty were statistically insignificant. The neurological outcome, assessed by the mRS and GOS, was not affected by cranioplasty timing. The P-values in the subgroups, early versus late, were statistically insignificant. In our institutional experience, the optimal timing for performing cranioplasty is dependent on the resolution of cerebral swelling.
Although the definition of early cranioplasty varies in the literature, early cranioplasty is commonly set at 90 days after DC.[
Surgical site infection is one of the main risks complicating cranioplasty and has a significant impact on the reported outcome and patient’s recovery. We recently published a paper with a cohort of our patients who underwent cranioplasty from subcutaneously preserved bone flaps in abdominal pockets.[
In the present study, the risk of postoperative hydrocephalus, necessitating EVD insertion or ventriculoperitoneal shunt dependency, between both groups was statistically insignificant. Undoubtedly, subsequent surgical procedures following cranioplasty can affect the overall neurological outcome and drastically increase the length of stay. Of note, hydrocephalus is a common complication after cranioplasty, and it was reported to be higher in patients undergoing early cranioplasty compared to patients undergoing late cranioplasty.[
Hydrocephalus may start to develop secondarily to the disturbance in the dynamics of cerebrospinal fluid.[
A systemic review of Xu et al. compared early versus late cranioplasty.[
Zheng et al. conducted a systematic review and a meta-analysis to investigate the effectiveness of early versus late cranioplasty.[
Seizure is a major well-established complication following cranioplasty. New-onset seizure following cranioplasty was estimated to be 5–6%.[
The risk of seizure among patients who underwent cranioplasty has been associated with multiple factors. Previous TBI, hemorrhagic stroke, or neurological sequelae before cranioplasty increase the risk of developing seizure.[
Timing of cranioplasty has been shown to be insignificant predictor for developing seizure following cranioplasty.[
A systematic review by De Cola et al. showed that motor improvement occurs in the early cranioplasty group, coupled with improved other parameters, such as cognition.[
In addition, extensive physical therapy postcranioplasty is essential to positively influence the motor component of patients. Being a major trauma center, a comprehensive rehabilitation program may be required while awaiting for cranioplasty. This serves to facilitate recovery and improve the occupational status of the patient. At our institute, all patients are referred to a physical therapy program within 48 h of admission for DC/cranioplasty to ensure early rehabilitation. Those patients are evaluated daily for motor improvement, functional capacity, and neurological outcome. In the present study, postoperative GOS and mRS between both groups were statistically insignificant, indicating a similar neurological outcome.
Finally, there are a few limitations that need to be acknowledged before interpreting the results of the current study. First, the retrospective design of the study renders it liable to selection and information bias. Second, as all patients had autologous cranioplasty, patients with nonautologous cranioplasty were excluded from the study, limiting the overall number. In addition, the risk of bone flap resorption could not be investigated as a possible postoperative complication of cranioplasty due to the variation in the measurement. However, innumerable efforts were taken to address the obstacles in the current study. Despite these limitations, the present study highlighted the importance of investigating the neurological outcome and postoperative complications from a comparative perspective.
CONCLUSION
The neurological outcome in patients who underwent early versus late cranioplasty is almost identical. The differences in the rates of overall postoperative complications between early versus late cranioplasty were statistically insignificant. These findings are consistent with the previous published literature. When performing cranioplasty, timing is of less significance. Therefore, other clinical parameters, influencing the neurological outcome and postoperative complications, should be taken into consideration. Further multicenter, prospective studies investigating the neuropsychological outcome pre/postoperatively in relation to cranioplasty timing are required.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alkhaibary A, Alharbi A, Abbas M, Algarni A, Abdullah JM, Almadani WH. Predictors of surgical site infection in autologous cranioplasty: A retrospective analysis of subcutaneously preserved bone flaps in abdominal pockets. World Neurosurg. 2020. 133: e627-32
2. Alkhaibary A, Alharbi A, Alnefaie N, Aloraidi A, Khairy S. Cranioplasty: A comprehensive review of the history, materials, surgical aspects, and complications. World Neurosurg. 2020. 139: 445-52
3. Alkhaibary A, Alharbi A, Khairy S.editors. In reply to the letter to the editor regarding “predictors of surgical site infection in cranioplasty: A retrospective analysis of subcutaneously preserved bone flaps in abdominal pockets. World Neurosurg. 2020. 139: 658
4. Archavlis E, Carvi Y Nievas M. The impact of timing of cranioplasty in patients with large cranial defects after decompressive hemicraniectomy. Acta Neurochir (Wien). 2012. 154: 1055-62
5. Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J. Cranioplasty after postinjury decompressive craniectomy: Is timing of the essence?. J Trauma. 2010. 69: 270-4
6. Bender A, Heulin S, Röhrer S, Mehrkens JH, Heidecke V, Straube A. Early cranioplasty may improve outcome in neurological patients with decompressive craniectomy. Brain Inj. 2013. 27: 1073-9
7. Bjornson A, Tajsic T, Kolias AG, Wells A, Naushahi MJ, Anwar F. A case series of early and late cranioplasty-comparison of surgical outcomes. Acta Neurochir (Wien). 2019. 161: 467-72
8. Bobinski L, Koskinen LO, Lindvall P. Complications following cranioplasty using autologous bone or polymethylmethacrylate-retrospective experience from a single center. Clin Neurol Neurosurg. 2013. 115: 1788-91
9. Borger V, Schuss P, Kinfe TM, Vatter H, Güresir E. Decompressive craniectomy for stroke: Early cranioplasty is a predictor for postoperative complications. World Neurosurg. 2016. 92: 83-8
10. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015. 23: 75
11. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
12. Cheng YK, Weng HH, Yang JT, Lee MH, Wang TC, Chang CN. Factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008. 15: 1115-9
13. Cho KC, Park SC, Choe IS, Seo DH. Safety and efficacy of early cranioplasty after decompressive craniectomy in traumatic brain injury patients. J Korean Neurotraumatol Soc. 2011. 7: 74
14. Chun HJ, Yi HJ. Efficacy and safety of early cranioplasty, at least within 1 month. J Craniofac Surg. 2011. 22: 203-7
15. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011. 364: 1493-502
16. Corallo F, De Cola MC, Lo Buono V, Marra A, De Luca R, Trinchera A. Early vs late cranioplasty: What is better?. Int J Neurosci. 2017. 127: 688-93
17. Corallo F, Marra A, Bramanti P, Calabrò RS. Effect of cranioplasty on functional and neuropsychological recovery after severe acquired brain injury: Fact or fake? Considerations on a single case. Funct Neurol. 2014. 29: 273-5
18. de Cola MC, Corallo F, Pria D, Lo Buono V, Calabrò RS. Timing for cranioplasty to improve neurological outcome: A systematic review. Brain Behav. 2018. 8: e01106
19. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9
20. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia. 1993. 34: 453-8
21. Hng D, Bhaskar I, Khan M, Budgeon C, Damodaran O, Knuckey N. Delayed cranioplasty: Outcomes using frozen autologous bone flaps. Craniomaxillofac Trauma Reconstr. 2015. 8: 190-7
22. Hopkins A, Garman A, Clarke C. The Research Unit of the Royal College of Physicians. The first seizure in adult life. Value of clinical features, electroencephalography, and computerised tomographic scanning in prediction of seizure recurrence. Lancet. 1988. 331: 721-6
23. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975. 305: 480-4
24. Junior AC, Filho PT, Gonçalves MP, Neto AA, Zanini MA. Cranioplasty: An institutional experience. J Craniofac Surg. 2018. 29: 1402-5
25. King MA, Newton MR, Jackson GD, Fitt GJ, Mitchell LA, Silvapulle MJ. Epileptology of the first-seizure presentation: A clinical, electroencephalographic, and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998. 352: 1007-11
26. Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: A review of 10 years and 258 cases. World Neurosurg. 2014. 82: e525-30
27. Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution’s experience with the complications of cranioplasty. Br J Neurosurg. 2013. 27: 629-35
28. Li G, Wen L, Yang XF, Zheng XJ, Zhan RY, Liu WG. Efficacy of large decompressive craniectomy in severe traumatic brain injury. Chin J Traumatol. 2008. 11: 253-6
29. Malcolm JG, Rindler RS, Ahmad FU. In reply: Early cranioplasty is associated with greater neurological improvement: A systematic review and meta-analysis. Neurosurgery. 2018. 83: E90
30. Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G. Early cranioplasty is associated with greater neurological improvement: A systematic review and meta-analysis. Clin Neurosurg. 2018. 82: 278-88
31. Malcolm JG, Rindler RS, Chu JK, Grossberg JA, Pradilla G, Ahmad FU. Complications following cranioplasty and relationship to timing: A systematic review and meta-analysis. J Clin Neurosci. 2016. 33: 39-51
32. Morton RP, Abecassis IJ, Hanson JF, Barber JK, Chen M, Kelly CM. Timing of cranioplasty: A 10.75-year single-center analysis of 754 patients. J Neurosurg. 2018. 128: 1648-52
33. Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. Neurology. 1997. 49: 991-8
34. Piedra MP, Nemecek AN, Ragel BT. Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int. 2014. 5: 25
35. Piedra MP, Ragel BT, Dogan A, Coppa ND, Delashaw JB. Timing of cranioplasty after decompressive craniectomy for ischemic or hemorrhagic stroke. J Neurosurg. 2013. 118: 109-14
36. Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scott Med J. 1957. 2: 200-15
37. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V. Cranioplasty after decompressive craniectomy: The effect of timing on postoperative complications. J Neurotrauma. 2012. 29: 1090-5
38. Song J, Liu M, Mo X, Du H, Huang H, Xu GZ. Beneficial impact of early cranioplasty in patients with decompressive craniectomy: Evidence from transcranial Doppler ultrasonography. Acta Neurochir (Wien). 2014. 156: 193-8
39. Spencer R, Manivannan S, Sharouf F, Bhatti MI, Zaben M. Risk factors for the development of seizures after cranioplasty in patients that sustained traumatic brain injury: A systematic review. Seizure. 2019. 69: 11-6
40. Thavarajah D, de Lacy P, Hussien A, Sugar A. The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection-a case series of 82 patients. Br J Neurosurg. 2012. 26: 78-80
41. van Donselaar CA, Schimsheimer RJ, Geerts AT, Declerck AC. Value of the electroencephalogram in adult patients with untreated idiopathic first seizures. Arch Neurol. 1992. 49: 231-7
42. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62
43. Wang H, Zhang K, Cao H, Zhang X, Li Y, Wei Q. Seizure after cranioplasty: Incidence and risk factors. J Craniofac Surg. 2017. 28: e560-4
44. Xu H, Niu C, Fu X, Ding W, Ling S, Jiang X. Early cranioplasty vs late cranioplasty for the treatment of cranial defect: A systematic review. Clin Neurol Neurosurg. 2015. 136: 33-40
45. Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Sharan AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: A systematic review. Neurosurgery. 2011. 68: 1124-30
46. Yao Z, Hu X, You C. The incidence and treatment of seizures after cranioplasty: A systematic review and meta-analysis. Br J Neurosurg. 2018. 32: 489-94
47. Yeap MC, Chen CC, Liu ZH, Hsieh PC, Lee CC, Liu YT. Postcranioplasty seizures following decompressive craniectomy and seizure prophylaxis: A retrospective analysis at a single institution. J Neurosurg. 2018. 131: 936-40
48. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M. Complications following cranioplasty: Incidence and predictors in 348 cases. J Neurosurg. 2015. 123: 182-8
49. Zhang G, Yang W, Jiang Y, Zeng T. Extensive duraplasty with autologous graft in decompressive craniectomy and subsequent early cranioplasty for severe head trauma. Chin J Traumatol. 2010. 13: 259-64
50. Zheng F, Xu H, von Spreckelsen N, Stavrinou P, Timmer M, Goldbrunner R. Early or late cranioplasty following decompressive craniotomy for traumatic brain injury: A systematic review and meta-analysis. J Int Med Res. 2018. 46: 2503-12