- Department of Neurosurgery, University of Virginia, Charlottesville, United States.
Correspondence Address:
Nisha Dabhi BA, Department of Neurosurgery, University of Virginia, Charlottesville, United States.
DOI:10.25259/SNI_561_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nisha Dabhi, Panagiotis Mastorakos, Jennifer D. Sokolowski, Ryan T. Kellogg, Min S. Park. Effect of drug use in the treatment of acute ischemic stroke: A scoping review. 19-Aug-2022;13:367
How to cite this URL: Nisha Dabhi, Panagiotis Mastorakos, Jennifer D. Sokolowski, Ryan T. Kellogg, Min S. Park. Effect of drug use in the treatment of acute ischemic stroke: A scoping review. 19-Aug-2022;13:367. Available from: https://surgicalneurologyint.com/surgicalint-articles/11810/
Abstract
Background: Drugs of abuse have been associated with ischemic stroke; however, the clinical presentation, outcomes, and treatment data in this population are limited. The overall safety and efficacy of thrombolytic therapy and thrombectomy in these patients remain unclear. This scoping review summarizes published complications and clinical outcomes in patients with recent abuse of cocaine, methamphetamine (MA), cannabis, decongestant, opioids, alcohol, and 3,4-methylenedioxymethamphetamine (MDMA) presenting with acute ischemic stroke.
Methods: We conducted a scoping review of the primary literature that assessed outcomes data of thrombolytic therapy or thrombectomy in drug users with acute ischemic stroke. We searched PubMed, Ovid Medline, and Web of Science. Demographic and stroke characteristics, treatment, complications, and clinical outcomes at last follow-up were collected and summarized.
Results: We identified 51 studies in this review. Drugs of abuse of interest were cocaine (14 studies), MDMA (one study), MA (eight studies), cannabis (23 studies), alcohol (two studies), decongestants (one study), and opioids (two studies). Clinical presentation and stroke presentation were most commonly described features. Thrombectomy outcomes were reported for four patients total (two studies), all with history of cocaine use. Thrombolysis treatment and outcomes were reported for 8851 patients (five studies) with history of cocaine, alcohol, or cannabis. Both treatments were pursued in three patients (three studies). Treatment complications included intracerebral hemorrhage, vasospasm, and cerebral edema.
Conclusion: Evidence for thrombolytic and thrombectomy treatment in drug users remains limited. Controlled studies are needed to examine complication profile and outcomes following thrombolytic and thrombectomy treatment in this population.
Keywords: Cerebrovascular accident, Drugs of abuse, Ischemic stroke, Thrombectomy, Thrombolysis
INTRODUCTION
Drug abuse has been a growing problem in the world associated with increased societal burden as well as short- and long-term health effects. According to the 2021 World Drug Report, the UN estimates that about 275 million people have used drugs worldwide annually with over 36 million people being classified as suffering from drug abuse disorders.[
Drugs of abuse have been found to weaken the integrity of the blood–brain barrier and affect vascular physiology. Specifically, several illicit drugs such as psychomotor stimulants – particularly amphetamine and cocaine – as well as opioids and psychotomimetic drugs have been found to cause vasospasm, vasculitis, accelerated atherosclerosis, and enhanced platelet aggregation.[
In addition, while current treatment for ischemic stroke includes thrombolysis and mechanical thrombectomy, drug users may be at increased risk of complications such as iatrogenic vasospasm and hemorrhagic reperfusion injury following these therapies as a consequence of the damaging physiological changes on blood vessels provoked by substances of abuse.[
Our scoping review aims (1) to describe the clinical presentation and treatment for patients with ischemic stroke associated with drugs of abuse including cocaine, MA, opioids, 3,4-methylenedioxymethamphetamine (MDMA), alcohol, and decongestants, and (2) to describe complications and outcomes related to thrombolysis therapy and thrombectomy in drug users, and (3) to identify areas for further research in the treatment of ischemic stroke in these patients.
MATERIALS AND METHODS
Search strategy and selection criteria
Electronic databases PubMed, Web of Science, and Ovid Medline were searched from inception to September 21, 2022. A keyword search using Boolean operators OR and AND with terms including but not limited to: “cocaine,” “amphetamine,” “cannabis,” “3,4-methylenedioxymethamphetamine,” “ephedrine,” “heroin,” “alcohol,” and “ischemic stroke” [Supplement for full search terms]. These substances were included in the literature review, as they have been known to affect the cerebrovasculature.
Inclusion and exclusion criteria were determined a priori. We included randomized control trials, observational cohort studies (prospective or retrospective), case–control studies, and case series that included (1) patients with drug abuse of the aforementioned substances who presented with acute ischemic stroke regardless of etiology with relevant clinical, treatment, or outcomes data. Studies were excluded if (1) they did not include clinical, treatment, or outcomes data for ischemic stroke associated with drugs of abuse, (2) they did not include positive toxicology for drug of abuse during presentation, and (3) they were not in the English language.
References of included articles were also reviewed for consideration to identify articles potentially missed by the electronic literature search [
Data extraction
The following baseline data were collected: patient sex and age; previous medical history of hypertension, diabetes, or prior cerebral infarct; stroke characteristics such as mechanism of ischemic stroke and stroke location; hospital admission data, including clinical presentation; treatment data, including type of therapy administered (i.e., tissue plasminogen activator (tPA), thrombectomy); stroke-related complications, including reperfusion injury, vasospasm, and cerebral edema; and clinical follow-up such as last follow-up time and outcome. Stroke-related complications were defined as complications occurring during hospital course attributable to either stroke treatment (i.e., tPA or thrombectomy) or natural progression of stroke (i.e., paralysis, locked-in syndrome, and cerebral edema).
Data synthesis
The study outcome was a descriptive assessment of the clinical presentation, complications, long-term morbidity, and mortality related to ischemic stroke in drug users as well as a descriptive assessment of outcomes related to thrombolysis and thrombectomy in this population. In included studies with multiple patients, continuous variables such as age and last clinical follow-up were reported as medians. Crude estimates of related variables were reported as the total proportion of all included studies.
RESULTS
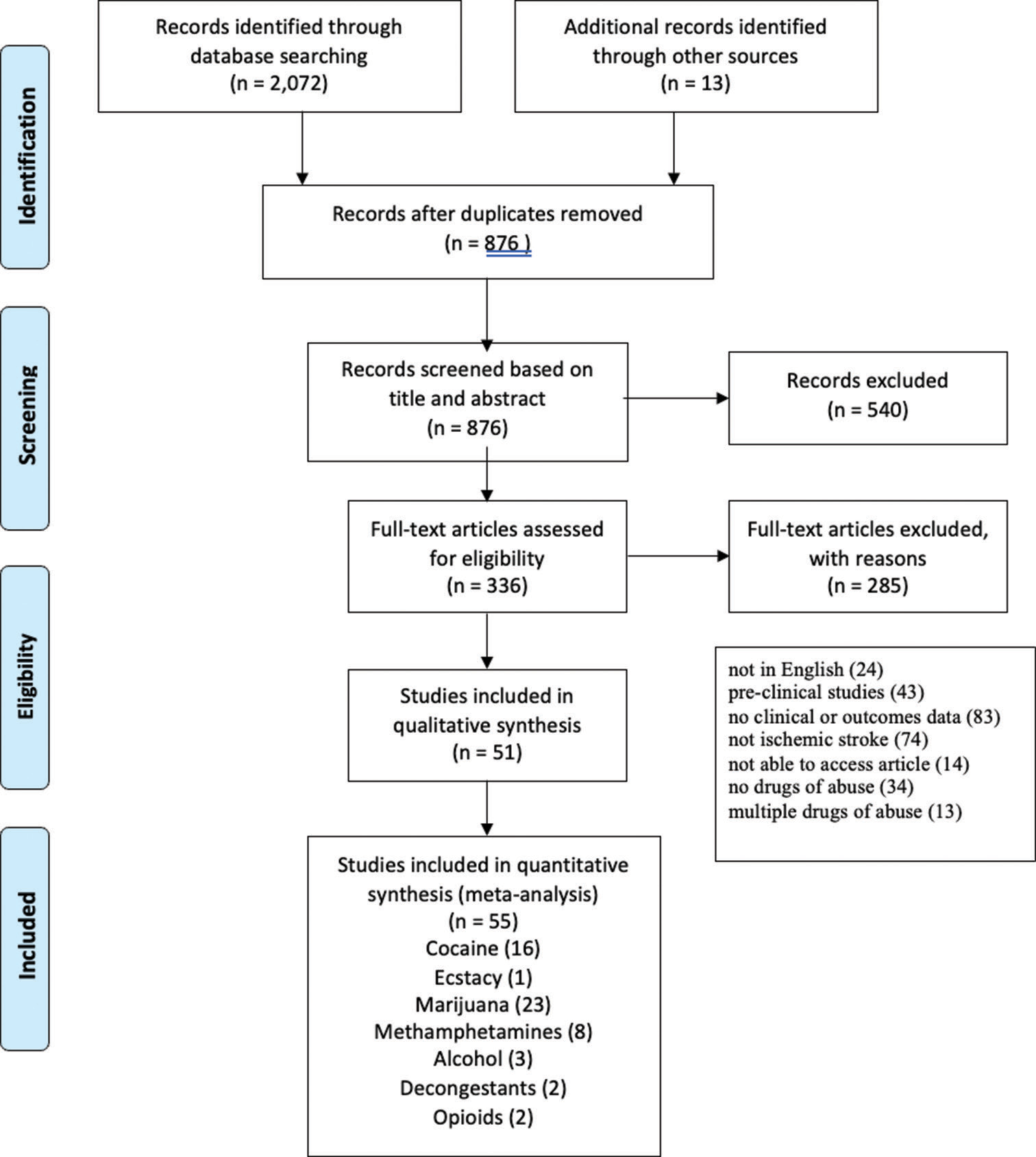
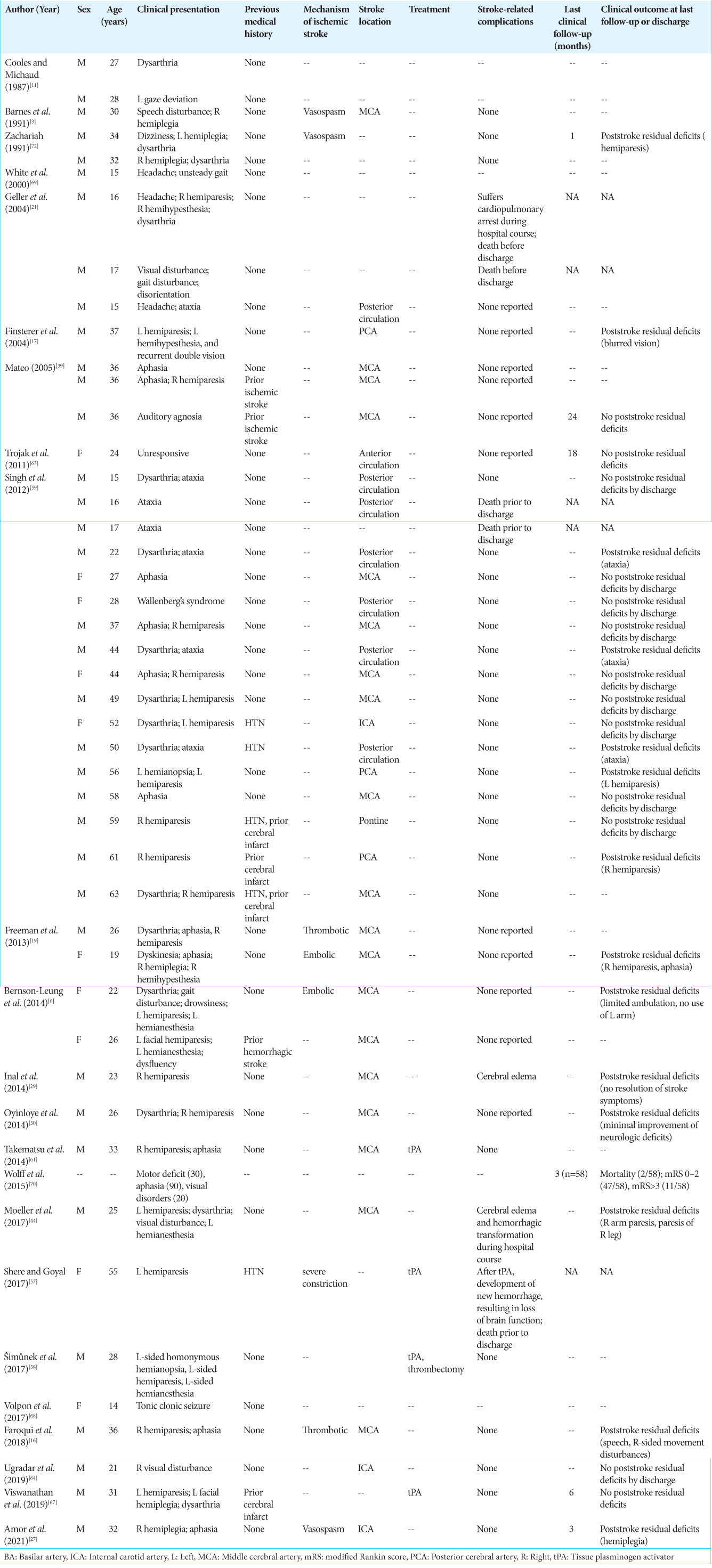
A total of 51 studies were included in this review. Baseline patient and stroke characteristics, imaging findings at admission, treatment, and clinical outcomes of each included study are summarized for each drug of abuse in
Cocaine
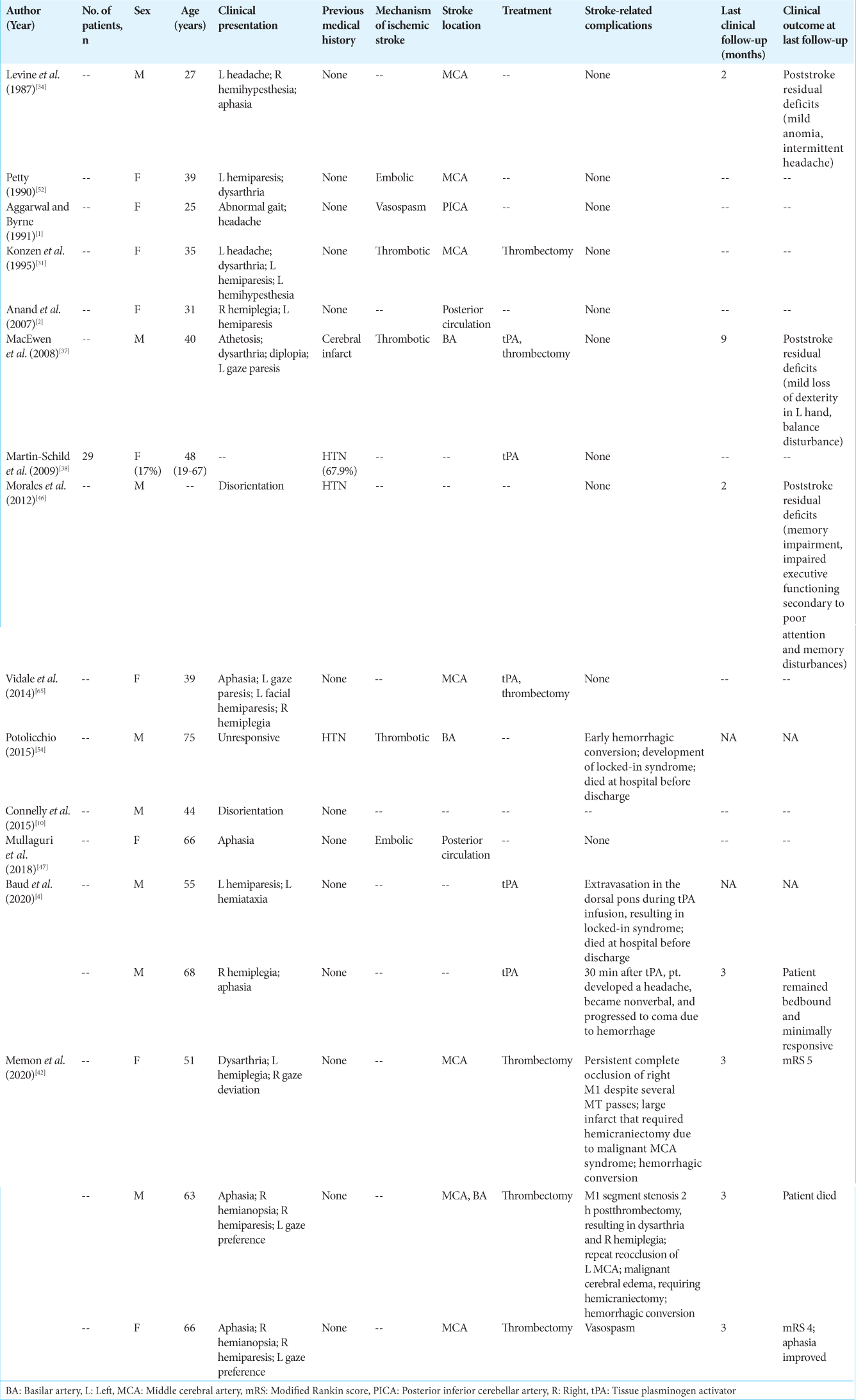
Fourteen studies with a total of 45 patients (13 females [29%]) reported on ischemic stroke in patients with a positive cocaine toxicology screen on presentation [
In six studies, patients with evidence of cocaine use were treated with either tPA and/or thrombectomy.[
In eight studies including eight patients, thrombectomy or tPA was not used for the treatment of ischemic stroke. Hemorrhagic conversion, resulting in death, occurred in one patient. Clinical follow-up (2 months) was available for two patients, at which time residual stroke deficits persisted.
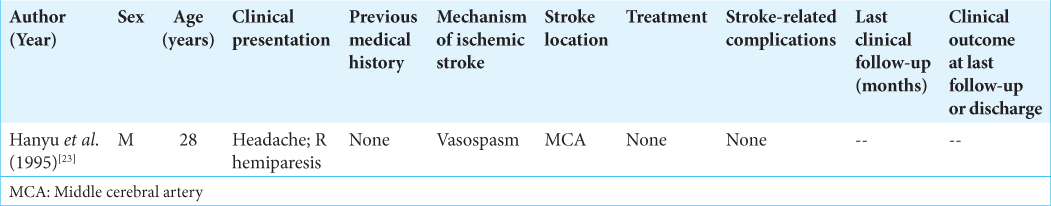
MDMA
Hanyu et al. presented one patient with MDMA-associated ischemic stroke [
Methamphetamine (MA)
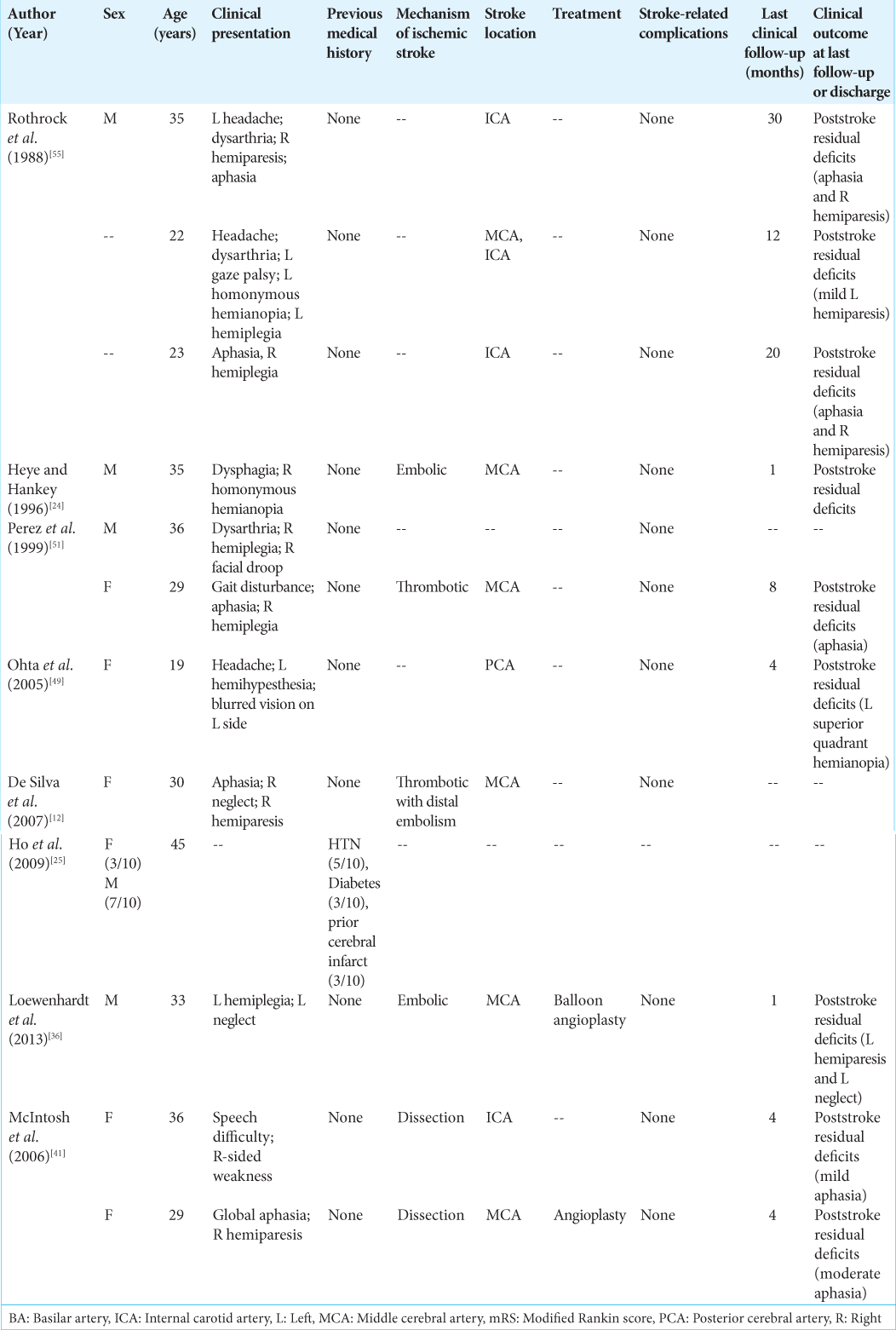
Eight studies described 21 patients [8 females (42%)] with a median age of 31.5 years [range, 19–45] presenting with acute ischemic stroke and MA use [
Two patients in two articles underwent invasive intervention for ischemic stroke.[
Cannabis
There were 23 studies identified that included patients presenting with cannabis induced ischemic stroke [
There were four case reports in which these patients were treated with thrombolysis or thrombectomy.[
Sixteen studies with a total of 39 patients reported complications in nonthrombolysis or thrombectomy treated strokes. Two patients developed cerebral edema and survived. Four patients died (cause unknown). In this cohort, clinical follow-up information was available for 26 patients (median time 6 [range 1–24]), of which 14 had residual deficits and 12 had no deficits.
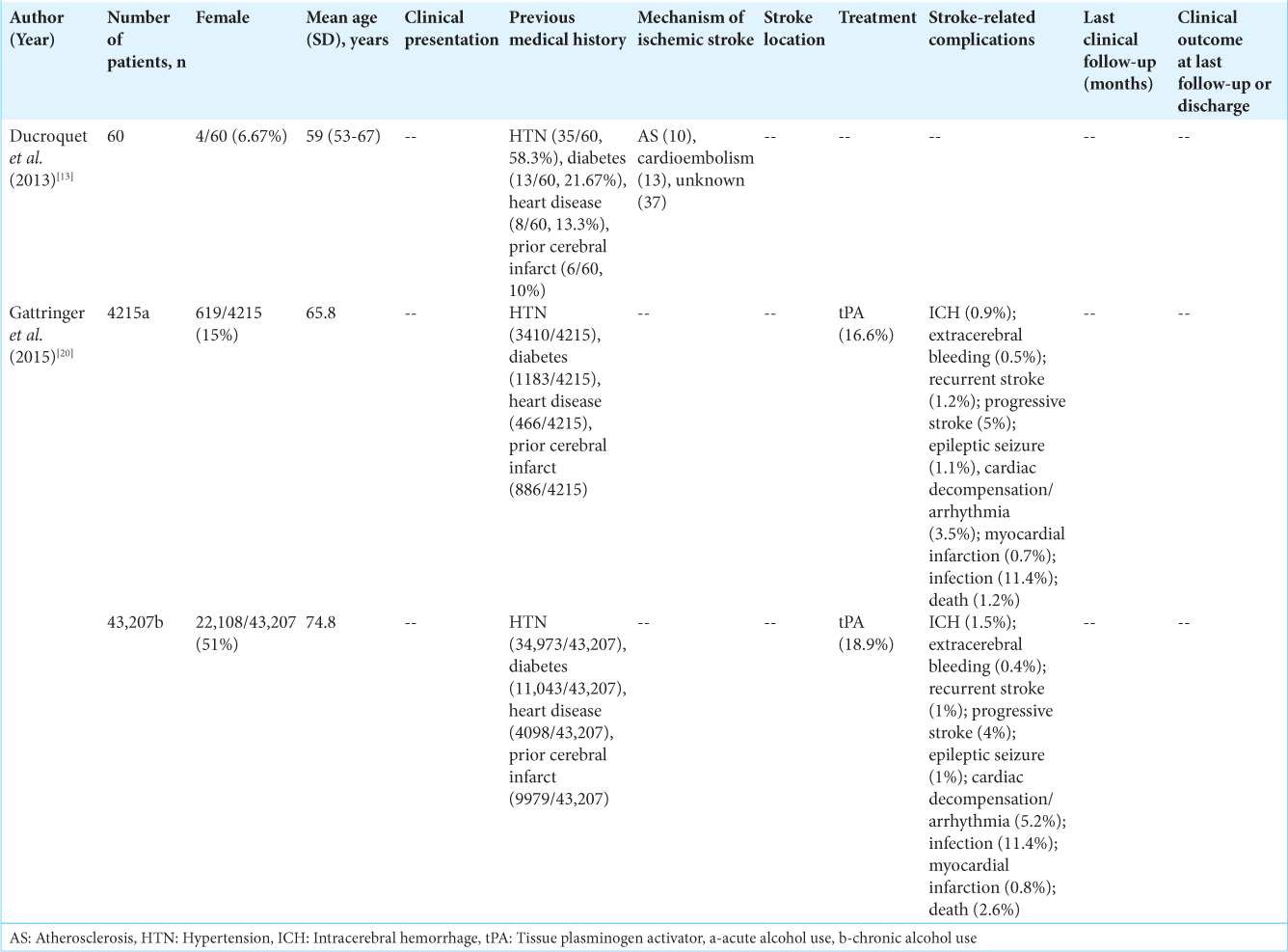
Alcohol
Two studies included clinical, treatment, or outcomes data related to ischemic stroke in patients with alcohol use [
Gattringer et al. compared ischemic stroke outcomes in 4215 and 43,207 of patients with a history of acute or chronic alcohol use and those without these factors, respectively.[
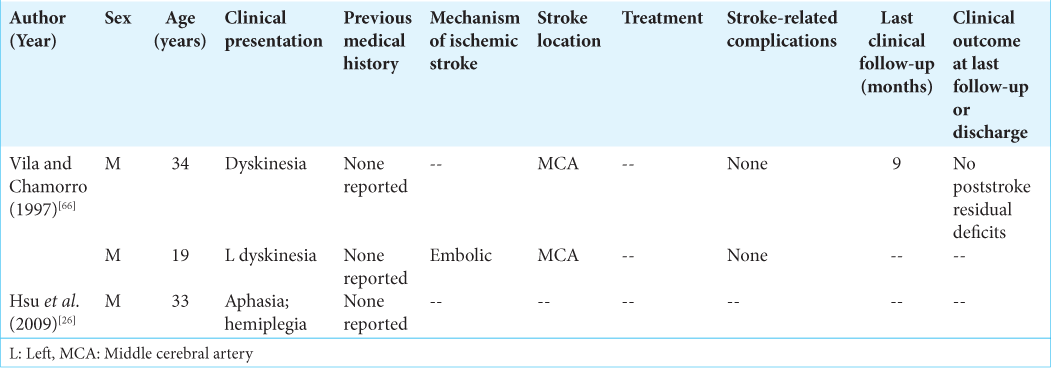
Opioids
There were two articles identified that described patients with evidence of opioid use presenting with acute ischemic stroke [
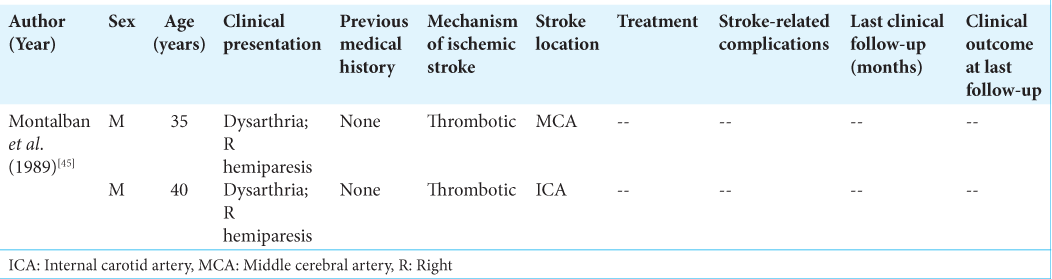
Decongestants
Montalban et al. described two male patients, aged 35 and 40, presenting with acute ischemic stroke and recent decongestant use [
DISCUSSION
In this scoping review, we present the clinical presentation, treatment, complication profile, and clinical outcomes related to ischemic stroke in patients with at least recent history of drugs of abuse. Data evaluating the clinical and stroke characteristics, complications, and outcomes in drug users presenting with ischemic stroke are lacking. While the average age of ischemic stroke is 69.6 years with most strokes occurring in the >65 age group, our review found that drug users with ischemic stroke were overall relatively younger, ranging from 28 years to 59 years, depending on the drug of abuse.[
However, while thrombolytic therapy and mechanical thrombectomy have revolutionized stroke care, their safety in drugs users is unclear. Risks occur with both thrombolytic therapy and mechanical thrombectomy. ICH and reperfusion injury can occur in up to 6% and 4.4% of patients undergoing tPA and endovascular thrombectomy, respectively.[
Thrombolysis and thrombectomy in cocaine users
Chronic cocaine use has been found to cause acute hypertensive episodes as well as cerebral small vessel disease, which are risk factors for intracranial bleeding.[
Cocaine use is also associated with induced vessel wall hyper-reactivity that can enhance the severity of iatrogenic vasospasm in the neurovasculature during mechanical thrombectomy.[
Thrombolysis and thrombectomy in amphetamine-type stimulant users
Amphetamines, such as MA, are psychostimulants that are associated with causing systemic hypertension and vasculitis, increasing baseline risk of intracranial bleeding compared to nonusers.[
Amphetamine use has also been associated with provoking endothelial dysfunction that perpetuates the development of iatrogenic vasospasm, similar to cocaine.[
Thrombolysis and thrombectomy in cannabis users
Chronic cannabis use can also induce dysfunction of the BBB by provoking transient arterial hypertension and inhibition of thrombin-driven clot formation that can increase risk of intracranial bleeding.[
Thrombolysis and thrombectomy in alcohol and opioid users
Alcohol has been found to compromise the structural integrity of the neurovasculature through disruption of the tight junctions and promotion of oxidative stress.[
Chronic alcohol consumption associated with reduced hepatic function has also been hypothesized to worsen clinical outcome following thrombolytic therapy based on animal model studies.[
In our review, we identified one study in which Gattringer et al. compared IV thrombolysis outcomes in patients with chronic or acute alcohol consumption to those without reported alcohol consumption.[
Limitations
We acknowledge that there are a number of limitations in this review. For one, our review was mostly limited to case reports with small sample sizes that did not detail all clinical data, treatment administered, complications, and clinical outcome with follow-up. There is also potential for selection bias with these studies that can compound the small sample sizes. Importantly, we were also unable to sub-stratify based on severity of stroke and presentation, chronic versus acute use of the respective drugs, and the quantitative amount of drug the patient consumed.
CONCLUSION
In this scoping review, we present the clinical presentation, outcomes, and treatment of ischemic stroke in drug users to highlight areas for further research. Data regarding clinical presentation and outcomes of ischemic stroke in drug users were available; however, evidence of the safety and efficacy of thrombolytic and thrombectomy treatment in these patients is severely lacking. The risk of hemorrhagic transformation and vasospasm as a result of these treatment modalities exist, yet the extent to which they are present in drug abusers, particular cocaine and cannabis users, is unknown. Controlled single and multicentered studies with large populations are needed to further examine complication profile and clinical outcomes data following thrombolytic and thrombectomy treatment in users of all drugs of abuse presented in this review.
Declaration of patient consent
Patients’ consent not required as there are no patients in this study .
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aggarwal S, Byrne BD. Massive ischemic cerebellar infarction due to cocaine use. Neuroradiology. 1991. 33: 449-50
2. Anand JS, Chodorowski Z, Wiśniewski M, Gólska A. A cocaine-associated quadriplegia and motor aphasia after first use of cocaine. Przegl Lek. 2007. 64: 316-7
3. Barnes D, Palace J, O’Brien MD. Stroke following marijuana smoking. Stroke. 1992. 23: 1381
4. Baud MO, Brown EG, Singhal NS, Hemphill JC. Immediate hemorrhagic transformation after intravenous tissue-type plasminogen activator injection in 2 cocaine users. Stroke. 2015. 46: e167-9
5. Beadell NC, Thompson EM, Delashaw JB, Cetas JS. The deleterious effects of methamphetamine use on initial presentation and clinical outcomes in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2012. 117: 781-6
6. Bernson-Leung ME, Leung LY, Kumar S. Synthetic cannabis and acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014. 23: 1239-41
7. Caprio FZ, Sorond FA. Cerebrovascular disease: Primary and secondary stroke prevention. Med Clin North Am. 2019. 103: 295-308
8. Carrino D, Branca JJ, Becatti M, Paternostro F, Morucci G, Gulisano M. Alcohol-induced blood-brain barrier impairment: An in vitro study. Int J Environ Res Public Health. 2021. 18: 2683
9. Coetzee C, Levendal RA, van de Venter M, Frost CL. Anticoagulant effects of a Cannabis extract in an obese rat model. Phytomedicine. 2007. 14: 333-7
10. Connelly KL, Chen X, Kwan PF. Bilateral hippocampal stroke secondary to acute cocaine intoxication. Oxf Med Case Reports. 2015. 2015: 215-7
11. Cooles P, Michaud R. Stroke after heavy cannabis smoking. Postgrad Med J. 1987. 63: 511
12. De Silva DA, Wong MC, Lee MP, Chen CL, Chang HM. Amphetamine-associated ischemic stroke: Clinical presentation and proposed pathogenesis. J Stroke Cerebrovasc Dis. 2007. 16: 185-6
13. Ducroquet A, Leys D, Al Saabi A, Richard F, Cordonnier C, Girot M. Influence of chronic ethanol consumption on the neurological severity in patients with acute cerebral ischemia. Stroke. 2013. 44: 2324-6
14. Esse K, Fossati-Bellani M, Traylor A, Martin-Schild S. Epidemic of illicit drug use, mechanisms of action/addiction and stroke as a health hazard. Brain Behav. 2011. 1: 44-54
15. Fanjun M, Junfa L, Bingxi Z, Fang J. nPKCepsilon and NMDA receptors participate in neuroprotection induced by morphine pretreatment. J Neurosurg Anesthesiol. 2006. 18: 119-24
16. Faroqui R, Mena P, Wolfe AR, Bibawy J, Visvikis GA, Mantello MT. Acute carotid thrombosis and ischemic stroke following overdose of the synthetic cannabinoid K2 in a previously healthy young adult male. Radiol Case Rep. 2018. 13: 747-52
17. Finsterer J, Christian P, Wolfgang K. Occipital stroke shortly after cannabis consumption. Clin Neurol Neurosurg. 2004. 106: 305-8
18. Fonseca AC, Ferro JM. Drug abuse and stroke. Curr Neurol Neurosci Rep. 2013. 13: 325
19. Freeman MJ, Rose DZ, Myers MA, Gooch CL, Bozeman AC, Burgin WS. Ischemic stroke after use of the synthetic marijuana “spice”. Neurology. 2013. 81: 2090-3
20. Gattringer T, Enzinger C, Fischer R, Seyfang L, Niederkorn K, Khalil M. IV thrombolysis in patients with ischemic stroke and alcohol abuse. Neurology. 2015. 85: 1592-7
21. Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics. 2004. 113: e365-70
22. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015. 372: 1019-30
23. Hanyu S, Ikeguchi K, Imai H, Imai N, Yoshida M. Cerebral infarction associated with 3,4-methylenedioxymethamphetamine (“ecstasy”) abuse. Eur Neurol. 1995. 35: 173-3
24. Heye N, Hankey GJ. Amphetamine-associated stroke. Cerebrovasc Dis. 1996. 6: 149-55
25. Ho EL, Josephson SA, Lee HS, Smith WS. Cerebrovascular complications of methamphetamine abuse. Neurocrit Care. 2009. 10: 295-305
26. Hsu WY, Chiu NY, Liao YC. Rhabdomyolysis and brain ischemic stroke in a heroin-dependent male under methadone maintenance therapy. Acta Psychiatr Scand. 2009. 120: 76-9
27. Ibn Hadj Amor H, Touil I, Boukriba S, Bouchnak S, Kraiem S, Rouabhia R. Case report: Spontaneous simultaneous coronary and carotid dissection in a young cannabis user. F1000Res. 2021. 10: 387
28. Ignaszewski MJ. The epidemiology of drug abuse. J Clin Pharmacol. 2021. 61: S10-7
29. Inal T, Köse A, Köksal O, Armagan E, Aydın SA, Ozdemir F. Acute temporal lobe infarction in a young patient associated with marijuana abuse: An unusual cause of stroke. World J Emerg Med. 2014. 5: 72-4
30. Krishnan R, Mays W, Elijovich L. Complications of mechanical thrombectomy in acute ischemic stroke. Neurology. 2021. 97: S115-25
31. Konzen JP, Levine SR, Garcia JH. Vasospasm and thrombus formation as possible mechanisms of stroke related to alkaloidal cocaine. Stroke. 1995. 26: 1114-8
32. Lappin JM, Darke S, Farrell M. Stroke and methamphetamine use in young adults: A review. J Neurol Neurosurg Psychiatry. 2017. 88: 1079-91
33. Lemarchand E, Gauberti M, de Lizarrondo SM, Villain H, Repessé Y, Montagne A. Impact of alcohol consumption on the outcome of ischemic stroke and thrombolysis: Role of the hepatic clearance of tissue-type plasminogen activator. Stroke. 2015. 46: 1641-50
34. Levine SR, Washington JM, Jefferson MF, Kieran SN, Moen M, Feit H. “Crack” cocaine-associated stroke. Neurology. 1987. 37: 1849
35. Liaw N, Liebeskind D. Emerging therapies in acute ischemic stroke. F1000Res. 2020. 9: v1000-546
36. Loewenhardt B, Bernhard M, Pierskalla A, Neumann-Haefelin T, Hofmann E. Neurointerventional treatment of amphetamine-induced acute occlusion of the middle cerebral artery by intracranial balloon angioplasty. Clin Neuroradiol. 2013. 23: 137-43
37. MacEwen C, Ward M, Buchan A. A case of cocaine-induced basilar artery thrombosis. Nat Clin Pract Neurol. 2008. 4: 622-6
38. Martin-Schild S, Albright KC, Misra V, Philip M, Barreto AD, Hallevi H. Intravenous tissue plasminogen activator in patients with cocaine-associated acute ischemic stroke. Stroke. 2009. 40: 3635-7
39. Mateo I, Pinedo A, Gomez-Beldarrain M, Basterretxea JM, Garcia-Monco JC. Recurrent stroke associated with cannabis use. J Neurol Neurosurg Psychiatry. 2005. 76: 435-7
40. Matsunaga Y, Morofuji Y, Horie N. Delayed vasospasm associated with mechanical thrombectomy for acute ischemic stroke. World Neurosurg. 2020. 138: 197-9
41. McIntosh A, Hungs M, Kostanian V, Yu W. Carotid artery dissection and middle cerebral artery stroke following methamphetamine use. Neurology. 2006. 67: 2259-60
42. Memon MZ, Kushnirsky M, Brunet MC, Siddu M, Starke RM, Malik AM. Mechanical thrombectomy for large vessel occlusions in cocaine associated acute ischemic stroke: Small case series and review of the literature. J Stroke Cerebrovasc Dis. 2020. 29: 105330
43. Mikami T, Obata R, Steinberg DI, Skliut M, Boniece I. Marijuana-related reversible cerebral vasoconstriction syndrome. Intern Med. 2021. 60: 795-8
44. Moeller S, Lücke C, Struffert T, Schwarze B, Gerner ST, Schwab S. Ischemic stroke associated with the use of a synthetic cannabinoid (spice). Asian J Psychiatr. 2017. 25: 127-30
45. Montalbán J, Ibañez L, Rodriguez C, Lopez M, Sumalla J, Codina A. Cerebral infarction after excessive use of nasal decongestants. J Neurol Neurosurg Psychiatry. 1989. 52: 541-3
46. Morales Vidal SG, Hornik A, Morgan C. Cocaine induced hippocampi infarction. BMJ Case Rep. 2012. 2012: bcr0320125998
47. Mullaguri N, Battineni A, Narayan A, Guddeti R. Cocaine Induced bilateral posterior inferior cerebellar artery and hippocampal infarction. Cureus. 2018. 10: e2576
48. Nawabi J, Kniep H, Schön G, Flottmann F, Leischner H, Kabiri R. Hemorrhage after endovascular recanalization in acute stroke: Lesion extent, collaterals and degree of ischemic water uptake mediate tissue vulnerability. Front Neurol. 2019. 10: 569
49. Ohta K, Mori M, Yoritaka A, Okamoto K, Kishida S. Delayed ischemic stroke associated with methamphetamine use. J Emerg Med. 2005. 28: 165-7
50. Oyinloye O, Nzeh D, Yusuf A, Sanya E. Ischemic stroke following abuse of Marijuana in a Nigerian adult male. J Neurosci Rural Pract. 2014. 5: 417-9
51. Perez JA, Arsura EL, Strategos S. Methamphetamine-related stroke: Four cases. J Emerg Med. 1999. 17: 469-71
52. Petty GW, Brust JC, Tatemichi TK, Barr ML. Embolic stroke after smoking “crack” cocaine. Stroke. 1990. 21: 1632-5
53. Pimentel E, Sivalingam K, Doke M, Samikkannu T. Effects of drugs of abuse on the blood-brain barrier: A brief overview. Front Neurosci. 2020. 14: 513
54. Potolicchio S. Cocaine-induced acute fatal basilar artery thrombosis: Report of a case and review of the literature. Am J Case Rep. 2015. 16: 393-7
55. Rothrock JF, Rubenstein R, Lyden PD. Ischemic stroke associated with methamphetamine inhalation. Neurology. 1988. 38: 589-92
56. Seo JW, Jones SM, Hostetter TA, Iliff JJ, West GA. Methamphetamine induces the release of endothelin. J Neurosci Res. 2016. 94: 170-8
57. Shere A, Goyal H. Cannabis can augment thrombolytic properties of rtPA: Intracranial hemorrhage in a heavy cannabis user. Am J Emerg Med. 2017. 35: 1988.e1-1988.e2
58. Šimůnek L, Krajina A, Herzig R, Vališ M. Cerebral Infarction in young marijuana smokers case reports. Acta Medica (Hradec Kralove). 2018. 61: 74-7
59. Singh NN, Pan Y, Muengtaweeponsa S, Geller TJ, Cruz-Flores S. Cannabis-related stroke: Case series and review of literature. J Stroke Cerebrovasc Dis. 2012. 21: 555-60
60. Siniscalchi A, De Sarro G, Pacifici R, Pisani E, Sanguigni S, Gallelli L. Thrombolytic therapy in cocaine users with ischemic stroke: A review of current practice. Psychopharmacol Bull. 2019. 49: 70-9
61. Takematsu M, Hoffman RS, Nelson LS, Schechter JM, Moran JH, Wiener SW. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin Toxicol (Phila). 2014. 52: 973-5
62. Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007. 83: 389-94
63. Trojak B, Leclerq S, Meille V, Khoumri C, Chauvet-Gelinier JC, Giroud M. Stroke with neuropsychiatric sequelae after cannabis use in a man: A case report. J Med Case Rep. 2011. 5: 264
64. Ugradar S, Manta A, Flanagan D. Unilateral cilioretinal artery occlusion following cannabis use. Ther Adv Ophthalmol. 2019. 11: 2515841419838661
65. Vidale S, Peroni R, Di Palma F, Sampietro A, Gozzi G, Arnaboldi M. Intra-arterial thrombolysis in a young patient with cocaine-associated stroke. Neurol Sci. 2014. 35: 1465-6
66. Vila N, Chamorro A. Ballistic movements due to ischemic infarcts after intravenous heroin overdose: Report of two cases. Clin Neurol Neurosurg. 1997. 99: 259-62
67. Viswanathan V, Yu C, Sambursky JA, Kaur S, Simpkins AN. Acute cerebral ischemia temporally associated with marijuana use. Cureus. 2019. 11: e5239
68. Volpon LC, Sousa CL, Moreira SK, Teixeira SR, Carlotti APC. Multiple cerebral infarcts in a young patient associated with marijuana use. J Addict Med. 2017. 11: 405-7
69. White D, Martin D, Geller T, Pittman T. Stroke associated with marijuana abuse. Pediatr Neurosurg. 2000. 32: 92-4
70. Wolff V, Schlagowski AI, Rouyer O, Charles AL, Singh F, Auger C. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: A potential mechanism involved in cannabis-related stroke. Biomed Res Int. 2015. 2015: 323706
71. Wolff V, Zinchenko I, Quenardelle V, Rouyer O, Geny B. Characteristics and prognosis of ischemic stroke in young cannabis users compared with non-cannabis users. J Am Coll Cardiol. 2015. 66: 2052-3
72. Zachariah SB. Stroke after heavy marijuana smoking. Stroke. 1991. 22: 406-9
73. Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H. Dose-related influence of chronic alcohol consumption on cerebral ischemia/reperfusion injury. Alcohol Clin Exp Res. 2011. 35: 1265-9