- Department of Neurosurgery, Hospital do Servidor Público Estadual, São Paulo, Brazil
Correspondence Address:
Matheus Fernandes de Oliveira
Department of Neurosurgery, Hospital do Servidor Público Estadual, São Paulo, Brazil
DOI:10.4103/2152-7806.175896
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Heringer LC, de Oliveira MF, José Marcus Rotta, Botelho RV. Effect of repeated transsphenoidal surgery in recurrent or residual pituitary adenomas: A systematic review and meta-analysis. Surg Neurol Int 08-Feb-2016;7:14
How to cite this URL: Heringer LC, de Oliveira MF, José Marcus Rotta, Botelho RV. Effect of repeated transsphenoidal surgery in recurrent or residual pituitary adenomas: A systematic review and meta-analysis. Surg Neurol Int 08-Feb-2016;7:14. Available from: http://surgicalneurologyint.com/surgicalint_articles/effect-of-repeated-transsphenoidal-surgery-in-recurrent-or-residual-pituitary-adenomas-a-systematic-review-and-meta-analysis/
Abstract
Background:Recurrent or residual pituitary adenomas previously treated by transsphenoidal surgery are not uncommon. There are no strongly established guidelines to perform treatment of such cases. The objective of this study is to elucidate the effect of transsphenoidal reoperation in residual or recurrent pituitary adenomas.
Methods:We made a systematic review of the literature to elucidate this effect through electronic search in MEDLINE/PubMed and Cochrane Central database. PRISMA statement was used as a basis for this systematic review and analysis of the risk of bias was made according to the Grading of Recommendations, Assessment, Development and Evaluation recommendations.
Results:In this review, fifteen studies were finally pooled analyzed. Although remission rates (RRs) and follow-up periods varied widely, from 149 patients with growth hormone-secreting tumors the mean RR was 44.5%, from 273 patients with adrenocorticotropic hormone-secreting tumors the mean RR was 55.5% and among 173 patients with nonsecreting tumors, RR was 76.1%. There was significant higher RR in nonsecreting tumors. Mean follow-up was 32.1 months. No difference was found between microscopic and endoscopic techniques.
Conclusions:A second transsphenoidal surgery is accompanied by a chance of remission in approximately half of cases with secreting tumors. In nonsecreting ones, success is higher.
Keywords: Endoscopy, microsurgery, pituitary adenoma, transsphenoidal, treatment
INTRODUCTION
Treatment of pituitary adenomas is often surgical, especially in hormone-secreting tumors or those with a mass effect on surrounding structures, such as the optic chiasm.[
In addition, it is well known that risks are higher in patients subjected to repeated transsphenoidal surgery than in patients without prior therapy.[
The objective of this study is to elucidate the effects of transsphenoidal reoperation for pituitary adenomas.
METHODS
A systematic review of the literature on the effects of repeated transsphenoidal surgery was performed using the MEDLINE (via PubMed) database and the Cochrane Central Register of Controlled Trials. The PRISMA statement and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) recommendations were followed.
Structure of the search strategy
The literature search was based on structured questions, which defined the criteria for inclusion of papers, based on types of participants (patients), intervention and control, follow-up, and types of studies, following the PICOT strategy.
Types of participants: Adult patients, of both genders, with pituitary adenoma, who experienced tumor recurrence or residual tumor after primary surgery and underwent revision surgery. Prolactinomas were excluded from evaluation due to the established concept of its treatment with dopaminergic agonists.
We excluded patients who either underwent radiotherapy (RT)/radiosurgery or who were receiving medical therapy to attain remission previously to the second transsphenoidal surgery.
Types of intervention/exposure: Transsphenoidal reoperation of pituitary adenomas.
Outcomes: The outcome selected for the study was the remission rate (RR) after second operation (reoperation).
Follow-up time or extraction of outcomes: We included all studies that reported follow-up time.
Types of study: We aimed to include clinical trials; if not possible, we accepted all cohorts. Case reports and studies evaluating <10 cases were excluded.
Language: Articles published in English, Portuguese, French, and Spanish from 1964 to 2014 were considered for analysis.
Analysis of risk of bias: The risk of bias was analyzed in accordance with the GRADE criteria.
Search strategies
The search strategy used is detailed below:
“Pituitary neoplasms” [MeSH Terms] OR “pituitary” [All Fields] AND “recurrence” [MeSH Terms] AND Transsphenoidal [All Fields] AND “surgery” [Subheading] OR “surgery” [All Fields] OR “surgical procedures, operative” [MeSH Terms] OR “surgical” [All Fields] AND “procedures” [All Fields] AND “operative” [All Fields] OR “operative surgical procedures” [All Fields] OR “surgery” [All Fields].
Searches were performed by two authors independently, and disagreements were resolved by discussion.
Statistics
RR was described as a percentage, mean, standard deviation, range, and confidence intervals. Follow-up time was described as mean, standard deviation, and range.
A meta-analysis of pooled data was made according to the type of tumor. The RRs of the surgical operated pituitary adenomas were compared across pituitary tumors types according to the method proposed by Neyeloff et al.[
A comparison between secreting and nonsecreting tumors was performed with Chi-square test.
RESULTS
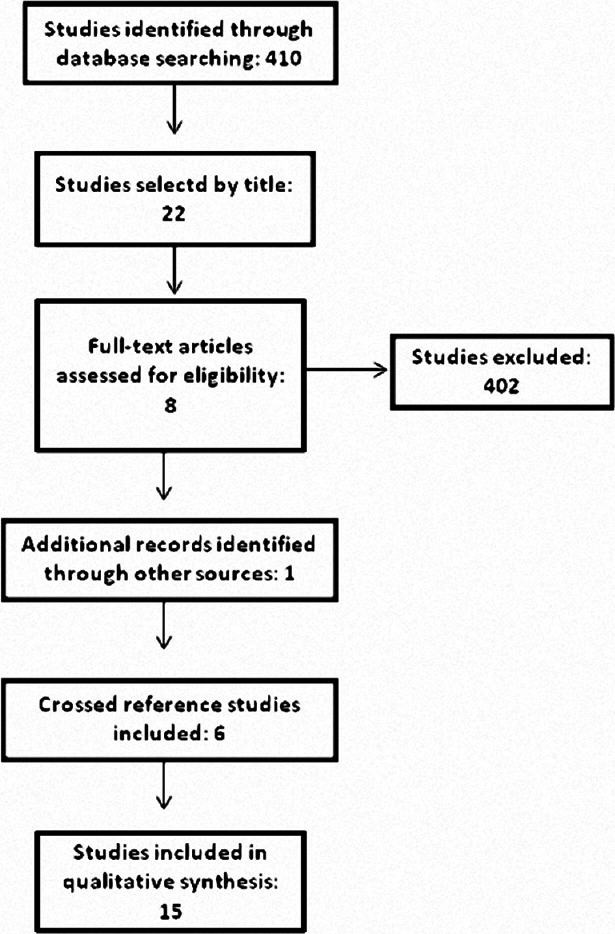
Search
The search strategy retrieved 410 articles [
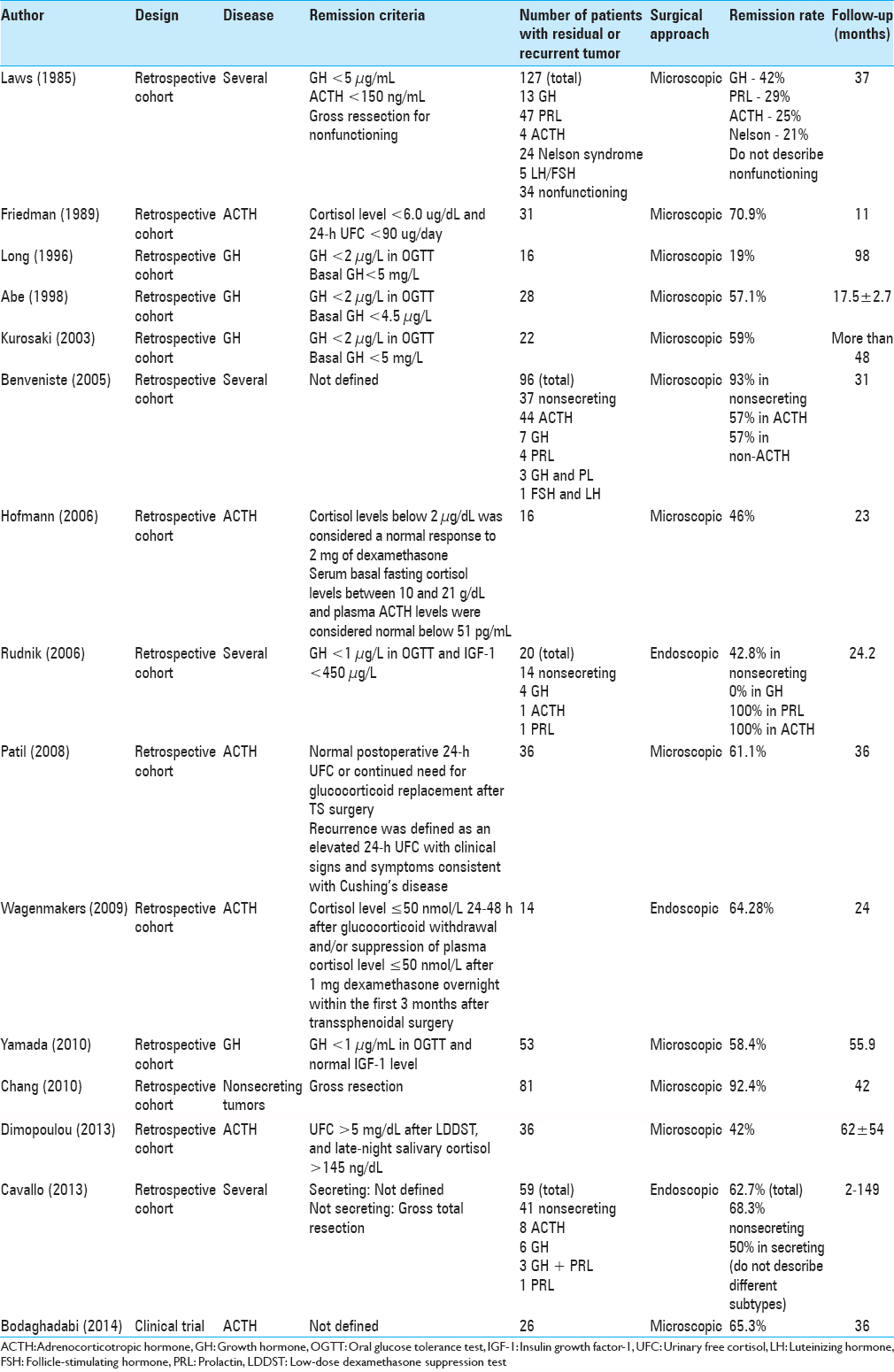
Data extraction [ Table 1 ]
Laws et al.[
Friedman et al.[
Long et al.[
Abe and Lüdecke[
Kurosaki et al.[
Benveniste et al.[
Hofmann et al.[
Rudnik et al.[
Patil et al.[
Wagenmakers et al.[
Yamada et al.[
Chang et al.[
Dimopoulou et al.[
Cavallo et al.[
Gross total resection was achieved in 37 cases (62.7%). On average, 38.4% of gross total resections left residual lesions which regrew (60.8% in nonsecreting adenomas, 15 of 24; 14.3% insecreting adenomas, 1 of 7); the recurrence rate was 74.6% (76.5% in nonsecreting adenomas, 13 of 17; 72.7% in secreting adenomas, 8 of 11).[
Bodaghabadi et al.[
Analysis of data pooled by tumor type
Growth hormone-secreting
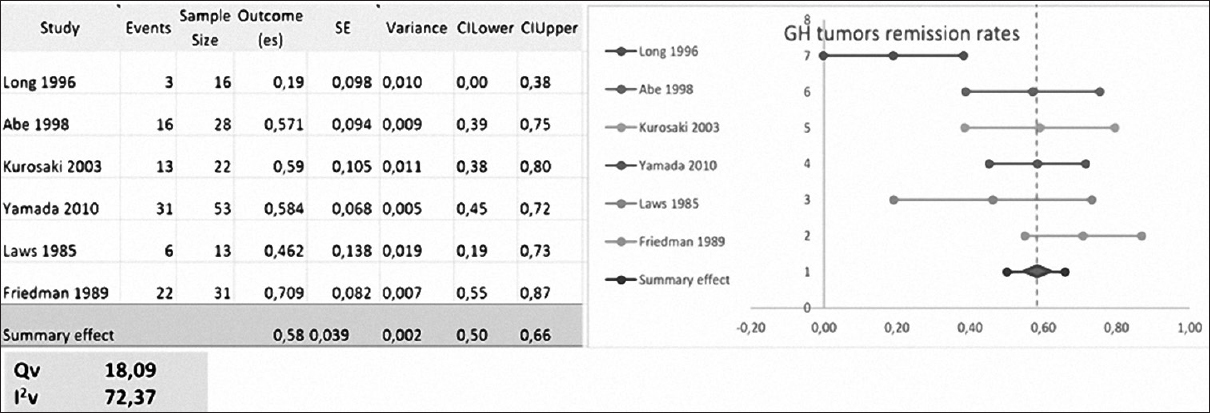
Yamada, Kurosaki, Rudnik, Cavallo, Benveniste, Abe, Laws, and Long included a total of 149 patients with residual or recurrent GH-secreting tumors. Data from Benveniste and Cavallo were not pooled due to a low number of studied patients.
Laws used basal GH levels as remission criteria. Long, Abe and Kurosaki used basal GH and GH levels after glucose tolerance test (GTT). Rudnik and Yamada used GH levels after GTT and insulin growth factor-1 levels [
The mean RR was 44.5 ± 23.85% (range, 0–72.8%) with repeated transsphenoidal surgery.[
Adrenocorticotropic hormone-secreting
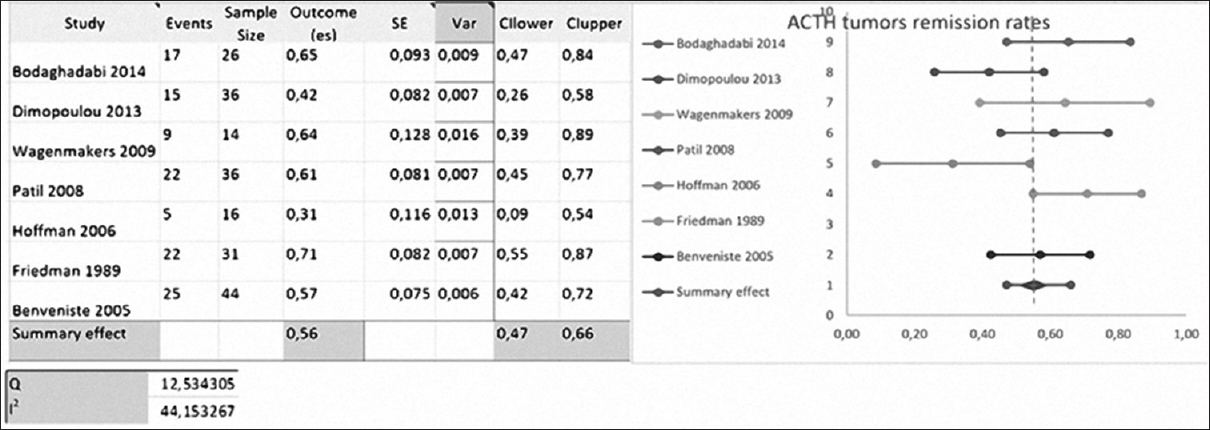
Hofmann, Patil, Bodaghabadi, Wagenmakers, Dimopoulou, Benveniste, Cavallo, Rudnik, Laws, and Friedman included 213 cases of recurrent ACTH-secreting tumors.
Laws used ACTH as remission criteria. Friedman, Hofmann, and Wagenmakers used basal cortisol levels and urinary cortisol levels as remissions criteria. Patil used urinary cortisol levels, and Dimopoulou used urinary and salivary cortisol levels [
The mean RR was 55.5 ± 15.89% (range, 25–72.7%) with repeated transsphenoidal surgery.[
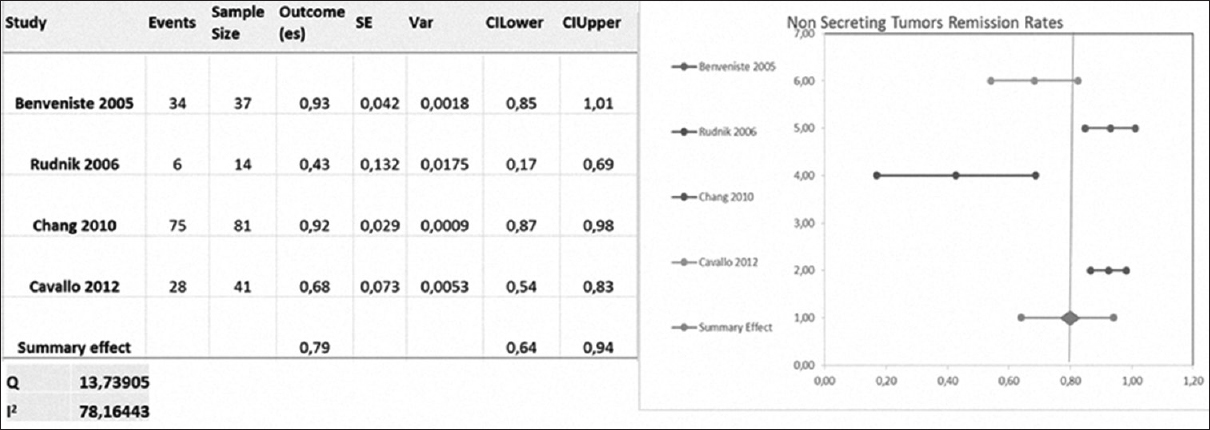
Nonsecreting tumors
Benveniste, Chang, Rudnik, and Cavallo included 173 nonsecreting tumors. Remission was defined as an image criteria revealing gross resection [
The gross resection rate was 76.05 ± 23.52% (ranging from 42.8% to 93%) with repeated transsphenoidal surgery [
Endoscopic versus microscopic surgery
Yamada, Kurosaki, Benveniste, Abe, Laws, Long, Hofmann, Patil, Bodaghabadi, Dimopoulos, Friedman and Chang reported transsphenoidal surgery using microscopic techniques. The mean RR reported was 56.5 ± 19.7%.[
Rudnik, Cavallo, and Wagenmakers published surgical series of repeated transsphenoidal surgery using the endoscopic technique.[
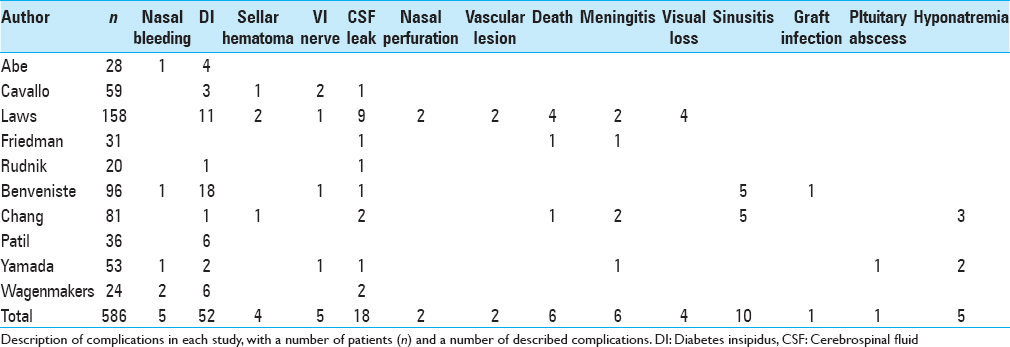
Complications in repeated transsphenoidal surgery
Among 15 studies evaluated, 3 did not even describe complications (Dimopoulou et al.; Long et al.; Bodaghabadi et al.) and 2 described no complications among their patients (Hofmann et al.; Kurosaki et al.). Finally, 10 studies described complications [
Synthesis of results
The rate of gross resection after repeated transsphenoidal surgery was approximately 76.05% for nonsecreting tumors. In secreting lesions, the average RR was 55.5% in ACTH-secreting and 44.5% in GH-secreting tumors. In short, RRs were higher in nonsecreting tumors than in secreting tumors (P = 0.001, Chi-square test).
Bias
Only one of the publications included was a randomized trial (Bodaghabadi et al.),[
Several studies evaluated homogeneous samples of recurrent tumors. However, Benveniste, Rudnik, Cavallo, and Laws evaluated a range of tumors but reported remission as an outcome in different subtypes.[
All studies described the type of intervention and the outcome measure evaluated in this study, i.e., the average rate of tumor remission.
The length of follow-up varied widely, from 2 to 149 months (mean, 32.1 months), and was significantly different across studies (Chi-square, P < 0.002). None of the studies reported the significant loss to follow-up. However, Chang et al. found that postoperative images were available for only 74.07% of patients.[
DISCUSSION
The second surgery is a common situation in the treatment of pituitary adenomas, which are lesions especially prone to recurrence or regrowth.[
Remission is defined differently in many of the quoted papers. Nevertheless, recurrence of pituitary tumors is a fact and must be addressed.
Currently, there is no guideline for the management of such cases, and treatment protocols are largely based on individual experience rather than on evidence-based literature. RT and radiosurgery, which are almost always reserved for cases of failed surgical treatment, are being increasingly applied to recurrent tumors, with encouraging results.[
In our literature review, we were able to compare the results of reoperation and radiosurgery in only one randomized trial, which included 26 patients in each arm.[
Stratification of tumors by hormone type revealed striking differences in RRs and prognosis. The average RR was 44.5%in GH-secreting tumors; in ACTH-secreting tumors, 55.5%; and in nonsecreting tumors, 76.05% (P = 0.001).
Although the main interest of any intervention with systematic review always will be randomized trials, still now several questions cannot be answered with this type of publications due to the lack of papers until this moment. Then, nonrandomized trials represent the best available evidence. The cohort studies have many potential biases that the data interpretation must be done with caution. As no comparative intervention was selected to be the scope of this review, the main concern in performing a retrospective meta-analysis, i.e. differences in compared populations may be initially disregarded. The data evaluation was done in this study still did not take into consideration that follow-up time could be an independent factor for lower RRs. This must be considered as a limitation when analyzing the results of this study.
In addition, remission criteria used in literature are distinct and varied among published papers describing tumoral recurrence [
In summary, the pooled RR in the published literature after transsphenoidal reoperation for residual or recurrent pituitary adenomas was 45.5% in GH-secreting tumors, 55.5% in ACTH-secreting tumors and 76.05% in nonsecreting tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abe T, Lüdecke DK. Recent results of secondary transnasal surgery for residual or recurring acromegaly. Neurosurgery. 1998. 42: 1013-21
2. Aghi MK. Management of recurrent and refractory Cushing disease. Nat Clin Pract Endocrinol Metab. 2008. 4: 560-8
3. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013. 84: 843-9
4. Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD. Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg. 2005. 102: 1004-12
5. Bodaghabadi M, Riazi H, Aran S, Bitaraf MA, Alikhani M, Alahverdi M. Repeated transsphenoidal surgery or gamma knife radiosurgery in recurrent cushing disease after transsphenoidal surgery. J Neurol Surg A Cent Eur Neurosurg. 2014. 75: 91-7
6. Cappabianca P, Alfieri A, Colao A, Cavallo LM, Fusco M, Peca C. Endoscopic endonasal transsphenoidal surgery in recurrent and residual pituitary adenomas: Technical note. Minim Invasive Neurosurg. 2000. 43: 38-43
7. Cavallo LM, Solari D, Tasiou A, Esposito F, de Angelis M, D’Enza AI. Endoscopic endonasal transsphenoidal removal of recurrent and regrowing pituitary adenomas: Experience on a 59-patient series. World Neurosurg. 2013. 80: 342-50
8. Chang EF, Sughrue ME, Zada G, Wilson CB, Blevins LS, Kunwar S. Long term outcome following repeat transsphenoidal surgery for recurrent endocrine-inactive pituitary adenomas. Pituitary. 2010. 13: 223-9
9. Clark K. Surgery for the recurrent pituitary adenoma. Clin Neurosurg. 1980. 27: 309-14
10. Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: An analysis of findings at repeated pituitary surgery. J Neurosurg. 2002. 97: 1343-9
11. Dimopoulou C, Schopohl J, Rachinger W, Buchfelder M, Honegger J, Reincke M. Long-term remission and recurrence rates after first and second transsphenoidal surgery for Cushing's disease: Care reality in the Munich Metropolitan Region. Eur J Endocrinol. 2013. 170: 283-92
12. Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: Defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014. 117: 445-57
13. Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB. Repeat transsphenoidal surgery for Cushing's disease. J Neurosurg. 1989. 71: 520-7
14. Hofmann BM, Hlavac M, Kreutzer J, Grabenbauer G, Fahlbusch R. Surgical treatment of recurrent Cushing's disease. Neurosurgery. 2006. 58: 1108-18
15. Kurosaki M, Luedecke DK, Abe T. Effectiveness of secondary transnasal surgery in GH-secreting pituitary macroadenomas. Endocr J. 2003. 50: 635-42
16. Laws ER, Fode NC, Redmond MJ. Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg. 1985. 63: 823-
17. Long H, Beauregard H, Somma M, Comtois R, Serri O, Hardy J. Surgical outcome after repeated transsphenoidal surgery in acromegaly. J Neurosurg. 1996. 85: 239-47
18. McLaughlin N, Eisenberg AA, Cohan P, Chaloner CB, Kelly DF. Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg. 2013. 118: 613-20
19. Meij BP, Lopes MB, Ellegala DB, Alden TD, Laws ER. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002. 96: 195-208
20. Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012. 5: 52-
21. Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER. Outcomes after repeat transsphenoidal surgery for recurrent Cushing's disease. Neurosurgery. 2008. 63: 266-70
22. Rudnik A, Zawadzki T, Galuszka-Ignasiak B, Bazowski P, Duda I, Wojtacha M. Endoscopic transsphenoidal treatment in recurrent and residual pituitary adenomas - First experience. Minim Invasive Neurosurg. 2006. 49: 10-4
23. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: Outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013. 98: 3190-8
24. Wagenmakers MA, Netea-Maier RT, van Lindert EJ, Timmers HJ, Grotenhuis JA, Hermus AR. Repeated transsphenoidal pituitary surgery (TS) via the endoscopic technique: A good therapeutic option for recurrent or persistent Cushing's disease (CD). Clin Endocrinol (Oxf). 2009. 70: 274-80
25. Yamada S, Fukuhara N, Oyama K, Takeshita A, Takeuchi Y. Repeat transsphenoidal surgery for the treatment of remaining or recurring pituitary tumors in acromegaly. Neurosurgery. 2010. 67: 949-56